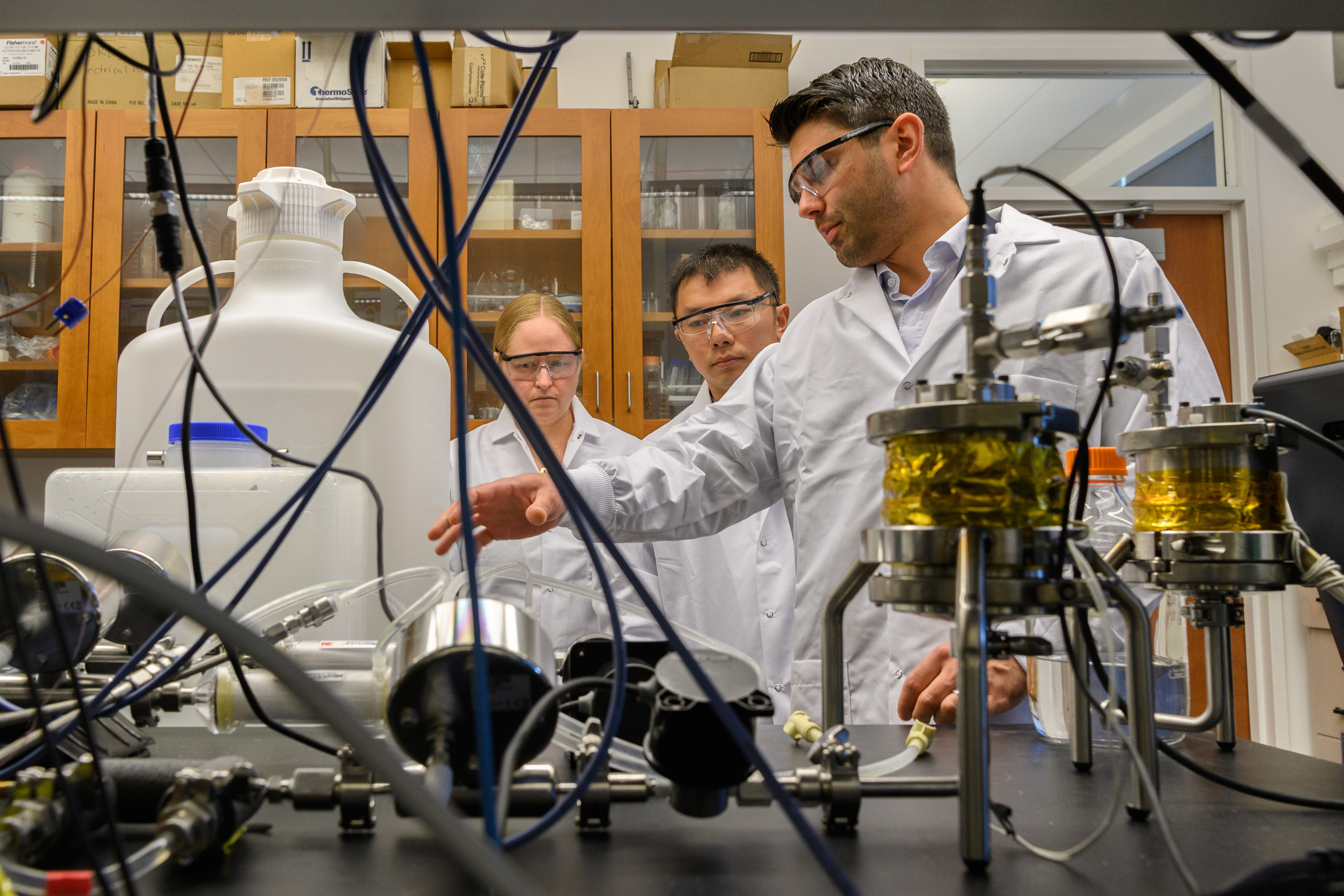
Researchers from UConn’s School of Pharmacy are leading a national effort to improve pharmaceutical manufacturing.
Research teams led by pharmaceutics professors Diane Burgess and Bodhisattwa “Bodhi” Chaudhuri are adapting the techniques of continuous manufacturing, which has long been used in electronics, chemical, and automobile manufacturing, to the production of complex drugs. With these techniques, drugs are made in a single uninterrupted process, unlike the batch-processing technology that is commonly used, in which medications are produced in a series of separate steps.
The new technologies being created at UConn could dramatically change the way today’s complex drug dosages are made, offering manufacturers a more responsive and reliable production platform to keep pace with the rapid advances taking place in medicine.

For years, drug makers have relied on decades-old batch-processing technology, in which medications are produced in steps, with many stops and starts along the way. But this method has had its share of problems, from errors caused by lapses in human supervision to production delays. Contamination risks are a constant concern, due to the open transfer of materials between stages. In some instances, whole batches of inferior or contaminated drugs have been thrown out, contributing to critical drug shortages and increasing costs for manufacturers and consumers alike.
The U.S. Food and Drug Administration has made improving pharmaceutical manufacturing a national priority. The FDA is counting on major academic research laboratories, like those in UConn’s School of Pharmacy, to create state-of-the-art manufacturing systems that can produce today’s complex new drug products quickly, reliably, and efficiently.
As part of that effort, researchers in UConn’s School of Pharmacy recently secured a highly competitive $3.3 million FDA grant to develop a continuous manufacturing platform for complex drug dosages that will eliminate many of the problems associated with batch processing of these products. The three-year grant recognizes UConn’s leadership in pharmaceutical manufacturing research, and was the first to be awarded under the 21st Century Cures Act, which was passed by Congress in 2016 to spur innovation in drug manufacturing and development.
“Complex drug products have unique characteristics that have greatly enhanced human health,” says Burgess, Board of Trustees Distinguished Professor of Pharmaceutics. “However, due to their complex formulation and processing requirements, their manufacturing cost is very high. Creating a more robust and modern production platform is absolutely essential if we are going to meet the growing demand for these important new products.”
The drug tablets and capsules that most of us are familiar with are considered simple dosage forms and are relatively easy to manufacture. Complex drug dosages, on the other hand, are often sophisticated precision medications that must be carefully produced as well as carefully administered.
They include a class of drugs known as parenteral preparations that cannot be taken orally. In some cases, these medications are rendered into nanosized particles such as liposomes that must be injected into the patient. Liposomes are made out of the same type of materials as the membranes of cells. They are widely used in HIV and cancer treatments because of their ability to travel to specific, targeted sites in the body – such as tumor locations – to release their medication, thereby reducing the occurrence of potentially harmful side effects.
But it is the very nature of these complex drugs – their sophistication and precision – that makes them a challenge to make at a commercial scale.
In continuous manufacturing, drugs are made in a single uninterrupted process from raw material to finished product. The system is highly automated, self-contained, and closed, significantly reducing the risk of human error and possible contamination. Multiple, built-in quality control features allow manufacturers to monitor quality during production, increasing efficiency.
In batch processing, quality control tests often are not performed until all of the steps in the process are complete, leading to significant production delays when entire batches are found to be inferior or contaminated and the process must be restarted.
The batch process is also ill-suited to new drug development, as manufacturers sometimes need to make significant adjustments in their production lines to accommodate new drug designs. Continuous manufacturing platforms are engineered to be more flexible, and would accelerate new drug development.
Continuous manufacturing is not new to Burgess’s lab. Her research team spent the past four years successfully developing a prototype continuous manufacturing platform for liposome-based drug products. This effort started with the thesis work of former Ph.D. student Antonio Costa, who is now an assistant research professor in the Burgess lab. With the support of UConn’s National Science Foundation I-Corps site, Accelerate UConn, the team is commercializing this platform. A patent for the technology is pending.
With the new FDA grant, Burgess and her team intend to leverage that technical foundation to create a more versatile modular continuous manufacturing platform that can be used to make a broader range of complex drugs.
In addition, a team led by co-principal investigator Chaudhuri, associate professor of pharmaceutics with expertise in chemical engineering and multi-scale computer modeling, is building multi-scale computer models that will predict the liposome size and encapsulation needed for a wide variety of drugs under different processing conditions (fluid flow rates, mixture concentrations, and temperature changes). Those predictions, along with experimental findings, will be systematically loaded into an extensive database. Information in the database will then be used to “train” an artificially intelligent (AI) computer system that will allow industry representatives and the FDA to clearly see how continuous manufacturing can be adopted for a multitude of complex pharmaceuticals.
Because the process is so new, federal regulations and policies regarding continuous manufacturing of complex drug products are currently limited. This has contributed to some manufacturers’ reluctance in adopting the technology, as it is largely untested and unregulated.
Chaudhuri says that in building the database and artificially intelligent neural network, the team will better understand the underlying molecular and fluid dynamics involved in the process, which are critical to making sure complex drug formulations are made properly.
“By using multi-scale computer models, we can see exactly how and where these nanoparticles and liposomes are being formed, which is almost impossible to observe or ascertain experimentally,” Chaudhuri says. “We can then run different simulations, varying such things as temperature, input pressure, velocity, or other factors until we get the precise outcomes – such as particle size distribution, concentration, and drug encapsulation.”
The AI network and database will allow users to input different drug elements, design parameters, and processing conditions to see how complex drugs can be made using continuous processing. It will serve as a road map for manufacturers seeking to adopt the new technology. It also will provide federal officials at the FDA with a clear scientific framework upon which to base new policies and regulations related to using continuous manufacturing for complex drug dosages.
“Developing a more robust and modern manufacturing technology that provides no interruption in production is essential to meeting today’s diverse therapeutic needs,” Burgess says. “The knowledge we will gain through this research will not only encourage future drug innovation, it should help guide federal authorities on future policy.”
Jie Shen, a former researcher in Burgess’s lab who is now an assistant professor of pharmacy at the University of Rhode Island, is also working on the continuous manufacturing project. Shen specializes in the development of advanced drug delivery systems such as those used in making complex dosages, and will test the prototype manufacturing system in her lab.
This project is funded by the federal Food and Drug Administration, grant #1U01FD005773-01.