Radenka Maric, UConn’s vice president for research, innovation and entrepreneurship, Dario Dekel from Technion’s Chemical Engineering Department and S. Pamir Alpay, UConn’s associate dean for research and industrial partnerships are working on advanced concepts that will provide novel solutions in catalysis and energy research.
UConn and Technion have had a relationship for two years under the UConn-Technion Energy Collaboration Initiative, which enables the exchange of faculty and students between the two schools for presentations and collaboration on research. The partnership was facilitated by UConn’s Office of Global Affairs.
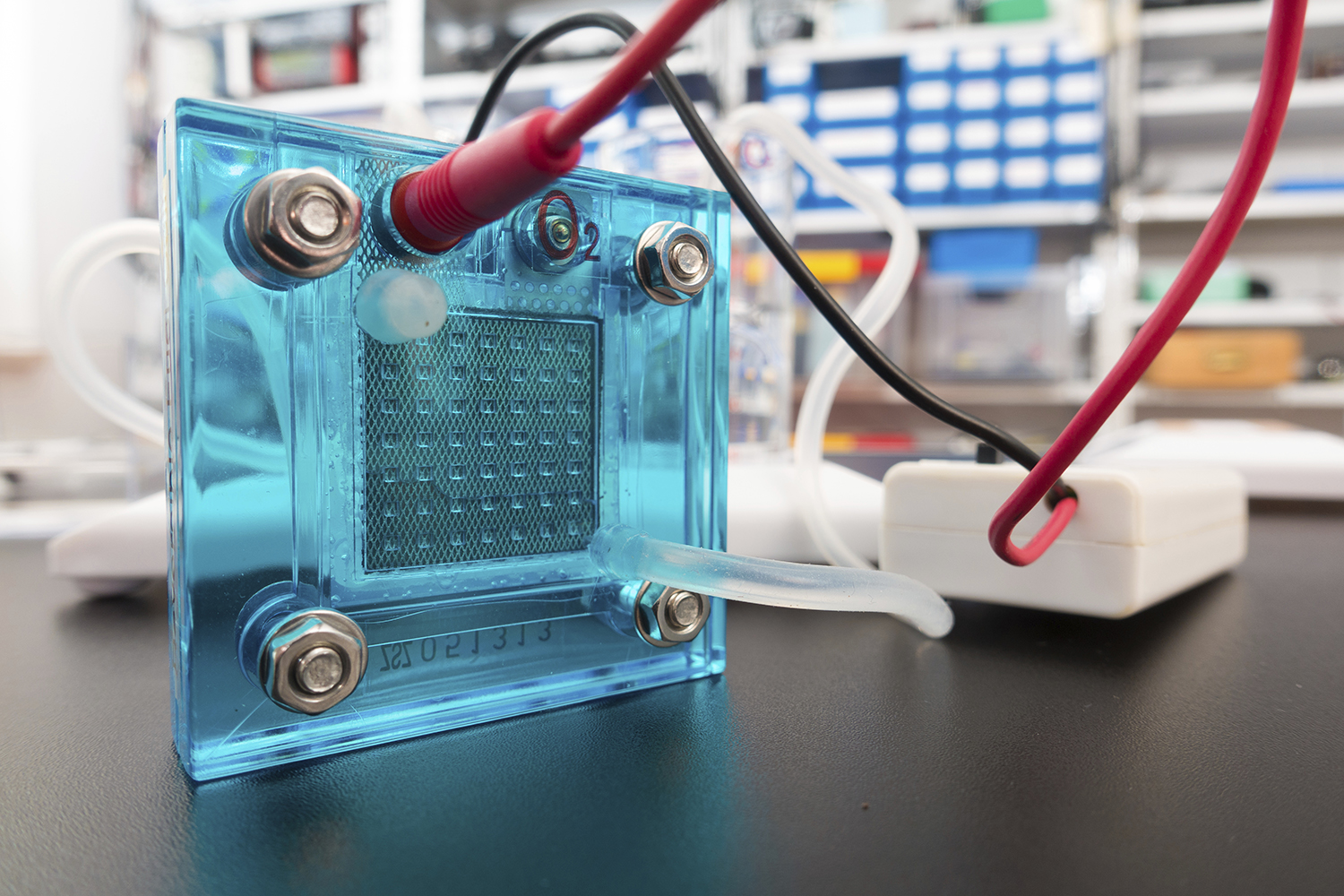
This initiative became the base for UConn and Technion to collaborate on several topics associated with the development of new materials and approaches to reduce precious metal content in anion-exchange membrane fuel cells (AEMFCs). These cells are remarkably efficient, but they are also expensive to manufacture right now.
This project focuses on fuel cells, an integral part of the clean energy initiative. Fuel cells use hydrogen and a catalytic layer to produce sustainable energy without emitting harmful greenhouse gases. These cells are commonly used for electric vehicles.
The current catalytic layer is incredibly expensive since it is made from a noble metal raw material: Platinum. This means highly efficient fuel cells are still prohibitively expensive in most cases. This is one of the biggest problems for clean fuel cell technology.
If scientists can reduce or eliminate the use of platinum in these cells, they would become much more affordable and easier to manufacture. This project is a potential game changer for fuel cell technology as it lays the groundwork for this to become a reality by replacing platinum with lower cost, more abundant metals like nickel and iron.
There are an increasing number of startups interested in this technology, as well as larger companies in the automotive industry. The success of UConn and Technion’s efforts could help propel this technology toward the mainstream.
“That would be a significant sign that we give to companies that this is the right time to make an investment in this technology,” Dekel says.
Alpay’s group is providing the theoretical background and the detailed analyses required for the understanding of physio-chemical mechanisms of the catalytic processes in such complex systems. This theoretical understanding will guide the group’s experiments.
Maric is responsible for developing the catalyst using a unique reactive spray deposition technology (RSDT), a technology she pioneered, for the synthesis of the novel materials systems. RSDT produces nanoparticles and allows for optimal conditions for catalysis.
Dekel and his group at Technion perfected the catalytic approach and the necessary measurement capabilities that are required to assess the efficiency of AEMFCs. They will conduct the testing of these materials.
The synergies between the UConn and Technion teams have provided the opportunity for a unique partnership.
“It’s a strong relationship,” Dekel says. “I can just pick the phone up and talk to them like we work together every day.”
For the past two years, UConn received seed money of $50,000 per year from Technion-Satell Foundation, which allowed the teams to work together seamlessly. To continue supporting this research, UConn has submitted a $500,000 proposal to the National Science Foundation (NSF). In conjunction, Technion applied for a proposal from the U.S.-Israel Binational Science Foundation (BSF) for the same project.