
The bacterium Escherichia coli, commonly known as E. coli, has a duplicitous reputation. Scientists tell us that most strains of the microbe live peacefully in our guts or the guts of other mammals, munching on bits of food, causing no harm or even creating benefits for their hosts.
But the grotesque imagery of E. coli infections tells a different story: After eating food contaminated with pathogenic strains, people can experience vomiting, diarrhea, and dysentery. And in rare cases, the bacteria can lead to kidney failure and even death.
Ken Campellone, assistant professor of molecular and cell biology in the College of Liberal Arts and Sciences, wants to understand how these bacteria can play such different roles. By focusing on the interactions between one of the deadliest E. coli strains and the cells of the human gut, he’s learning not only how the bacteria works, but how our own cells work, too.
Recently, Campellone discovered a particular protein in the cells of the human large intestine that is taken over by E. coli cells and helps to bind the bacterium to the intestinal wall.
“Pathogens have found really clever ways of taking over the normal processes of our cells,” he says. “Often they know more about our own cells than we do, and it’s really intriguing.”
The strain of E. coli that Campellone studies belongs to a group of the bacteria called enterohemorrhagic E. coli, or EHEC, that often makes international news when people eat contaminated meat or vegetables. In 2011, an outbreak of a hemorrhagic strain in Germany infected more than 3,700 people, killing 45. The Centers for Disease Control and Prevention estimate that about 75,000 infections occur each year in the United States.
The reason for this high level of virulence, says Campellone, is a series of genetic acquisitions by the harmful bacteria. Scientists have sequenced several types of E. coli, and they’ve found more than 1,000 genes in the harmful group that are not present in the harmless, or commensal, group.
But, he adds, of the roughly 1,000 genes that have been identified as pathogenic, relatively few have been characterized.
“We know very little about the genes in EHEC that are different from the commensal version,” he says. “My goal is to better understand how a group of genes that encode proteins called effectors take over their human cell targets.”
In particular, the most dangerous types have acquired the genes to produce a poisonous substance called Shiga toxin, which Campellone says can produce an illness ranging from unpleasant to life-threatening.
“If the toxin is just released into your intestines, you would get diarrhea and dysentery,” he says. “But if it enters your bloodstream, it can cause serious kidney damage and become fatal.” Plus, he adds, there are currently no known medicines for the blood poisoning syndrome, and antibiotics only make the symptoms worse. Patients just have to wait and hope.
Campellone’s research focuses on how the trafficking and organization of proteins control the shape of cells. When E. coli affix themselves to the intestinal wall, they disrupt its normal organization. They do this by delivering bacterial proteins into the cell, which in turn recruit specific intestinal cell proteins that normally shape the cell.
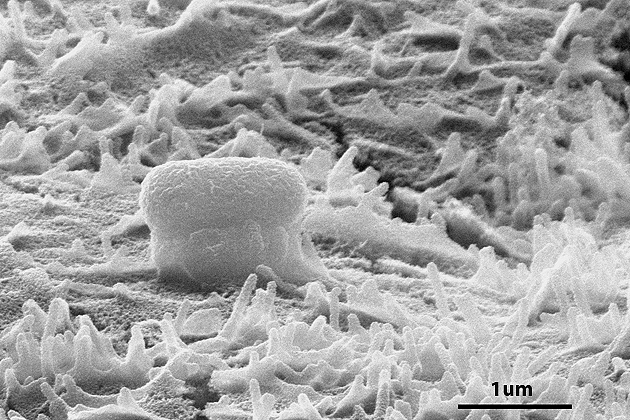
In 2004, Campellone was the first to identify a protein that the E. coli injects into the intestinal cells, causing the production of a fleshy bulge that lifts the attached bacteria away from the wall. Scientists call this lump a “pedestal” – because it really does look like one – but they still aren’t sure what its purpose is.
Campellone also recently discovered a protein in human intestinal cells that interacts with the bacterial protein to help create the pedestal. He published these results in the June 2012 issue of The Journal of Biological Chemistry.
The discovery is significant because if a drug was developed that could block the pedestal from being produced, then the E. coli might not be able to stick to the intestinal wall, he explains. In that case, the bacteria might simply wash through a person’s system, causing little harm.
In the classroom and in his laboratory, Campellone says these examples from his research give his students real-life examples of the information they learn about cell structure.
“When we teach cell biology, we show students that a lot of what we know about how human cells normally function is from studying infections,” he says, pointing out that many cellular proteins have only been discovered in the context of pathogens trying to exploit them.
“Being able to ask scientific questions experimentally in the laboratory and then get an answer that could benefit people – that’s the most exciting part for the students, and for me,” he says. “You can be the first person in the world to know something.”


