Every expecting parent hopes to have a healthy, happy baby. But if a baby is born with a significantly smaller-than-normal head, it’s a sign that something is seriously wrong.
Rahul Kanadia, an associate professor in the University of Connecticut’s Department of Physiology and Neurobiology has received more than $1.8 million from the National Institutes of Health to study the role of minor spliceosome in cortical development common in microcephaly.
Microcephaly is a birth defect where a baby’s head is much smaller than usual at birth. This is a sign that the baby’s brain did not develop properly during the pregnancy. This condition can lead to seizures, developmental delays, intellectual disabilities, problems with movement and balance, trouble swallowing, hearing loss, and vision problems.
This life-threatening defect can be caused by inherited genetic mutations or the Zika virus. Zika is spread by mosquitoes and can be transferred from infected pregnant women to their babies; the virus became an international Public Health Emergency in 2016 following its outbreak in Brazil, which eventually spread to the United States.
There is currently no treatment for this rare, life-long affliction that affects two to 12 babies per every 10,000 births in the United States. The risk is much higher, however, for babies born to mothers who are infected by the Zika virus, as high as 13 percent. These children require careful monitoring and increased health care for their entire lives.
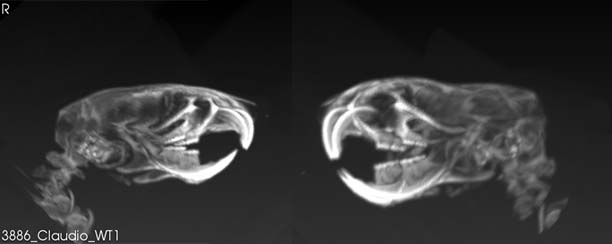
Kanadia’s project will use mouse models to look at the underlying molecular and cellular changes that occur during prenatal development that alter the process of normal cortical development in the baby’s brain and lead to microcephaly.
The UConn team discovered through prior research that the inactivation of the minor spliceosome results in microcephaly in mice. The minor spliceosome is a small nuclear RNA and protein complex that stimulates the removal of certain introns, non-coding regions of our genome, from messenger RNAs in order for them to be translated into proteins that carry out the essential functions of all cells.
Kanadia will test the hypothesis that minor spliceosome act in a cell-type specific manner to inform cell cycle regulation and neuron production— in effect, essentially allowing for normal development of the cortex.
“Long before the Zika-associated microcephaly was discovered, we were studying the importance of cell cycle regulation in proper cortical development,” explains Kanadia. “This surprising connection of our research to a newly emerging threat to proper cortical development underscores the importance of basic science research. Often, the relevance of basic science research is lost when one seeks to find an immediate health-related benefit. Our work shows that generating foundational knowledge on normal gene regulation in proper cortical development prepares us to better deal with emerging pathologies in the future.”
Kanadia’s research focuses on understanding the role of posttranscriptional gene regulation during vertebrate development with a specific focus on the role of minor intron splicing and alternative splicing.
This grant is NIH number: 1R01NS102538-01A1.