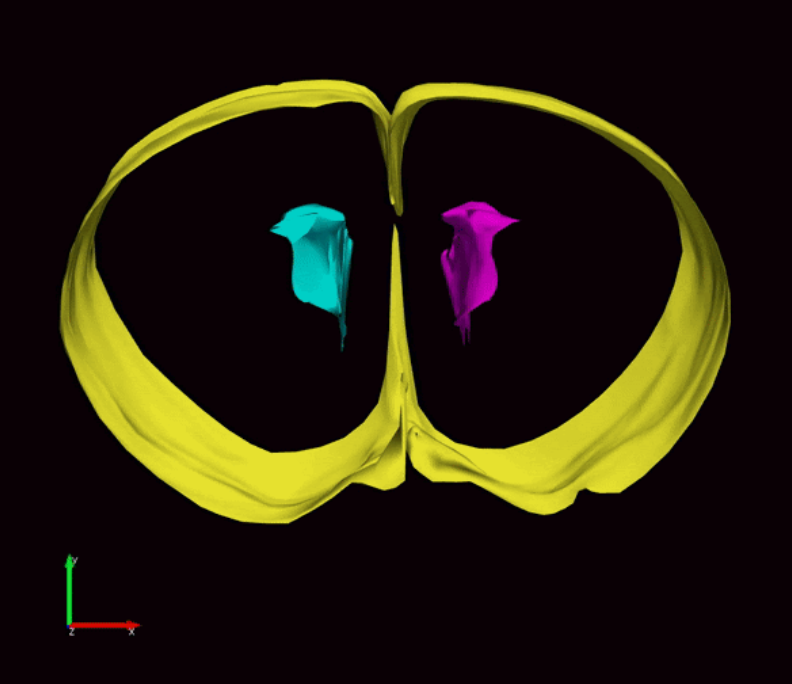
Pediatric hydrocephalus is a rare condition resulting from an excess of cerebrospinal fluid in the ventricles of the brain. Hydrocephalus affects one out of every 770 babies and is the most common reason infants require brain surgery.
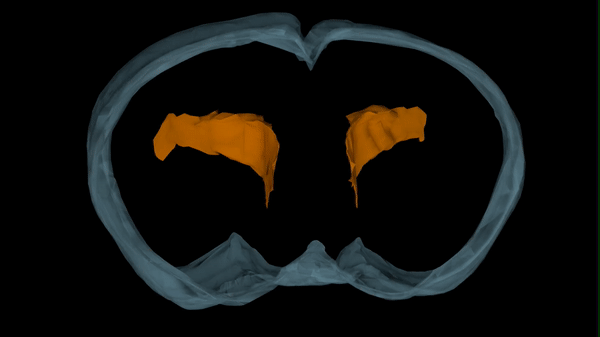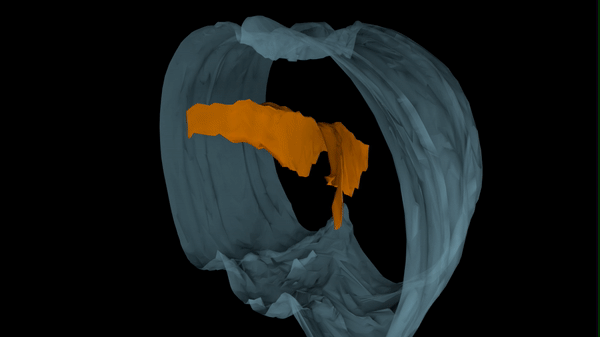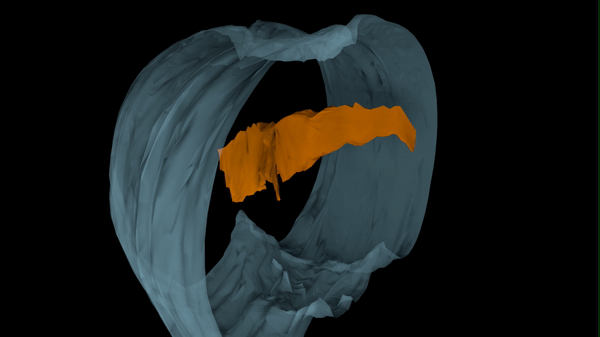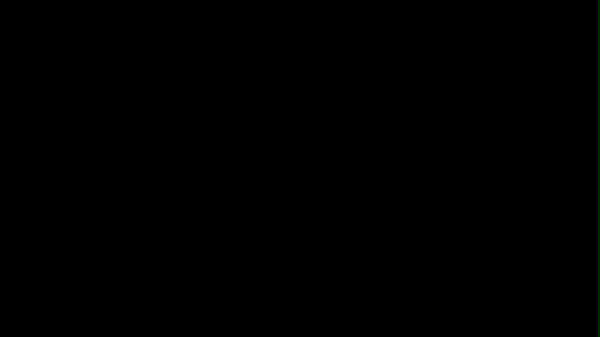
Hydrocephalus has numerous causes, one of which is viral and bacterial infections. While we know these kinds of infections can lead to hydrocephalus, the pathological progression that results in ventricle expansion (hydrocephalus) and altered brain development has not been clearly defined.
University of Connecticut professor of physiology and neurobiology Joanne Conover together with virologist Paulo Verardi, an associate professor of pathobiology and veterinary science, will lead a study looking at the impact of postinfectious hydrocephalus on the stem cell niche of the ventricular system during brain development. This work is supported by a $1.7 million grant from the National Institute of Neurological Disorders and Stroke.
During fetal development, stem cells line the ventricular system, hollow cavities within the brain filled with cerebrospinal fluid. The stem cells are responsible for the generation of new neurons and glial cells that populate the developing brain. Stem cells along the ventricles also generate a protective layer of ependymal cells which produce cerebrospinal fluid and provide barrier and transport functions between the cerebrospinal fluid and interstitial fluid surrounding brain tissue.
As ependymal cells form along the ventricle walls, the remaining stem cells are relegated to the layer below this barrier. These stem cells only remain until humans are about two-years-old but last a lifetime in rodents. Conover’s research will investigate the effect postinfectious hydrocephalus has on the critical period of brain development from fetal/embryonic to early postnatal development.
Certain viruses, like influenza, preferentially target the ependymal cell lining of the ventricles. This results in a loss of structural support and barrier functions provided by the ependymal cells. If a baby becomes infected during periods of neurogenesis and ependymogenesis, this would have a critical impact on stem cell function and ultimately brain development.
In their study, Conover and Verardi will model human influenza infection using mouse models. Together with their research teams, they will characterize the damage to and repair mechanisms provided by the ventricular-subventricular zone stem cells over the course of infection and resulting hydrocephalus.
The team will test the hypothesis that enlarged ventricles, which result from influenza infection, impact stem cell niche functions, compromise neurogenesis and result in perturbed brain development. The group will infect embryonic and postnatal mice with either a component of the influenza virus known to cause hydrocephalus or a mouse-specific version of influenza to more accurately replicate postinfectious hydrocephalus as seen in infants.
Development of a bona fide post-infectious hydrocephalus model in mice will allow detailed investigation of hydrocephalus progression, identify critical timepoints in neural development and highlight stem cells’ capacity for regenerative repair following infection.
The researchers will also test if prior immunity to viruses like influenza can limit the effects of post-infectious hydrocephalus.
“These studies will define the impact that post-infectious hydrocephalus has on a critical stem cell niche and its capacity for regenerative repair,” Conover says. “They will also guide treatment strategies for post-infectious hydrocephalus.”
This project is NIH Grant No.: 1R01NS110586