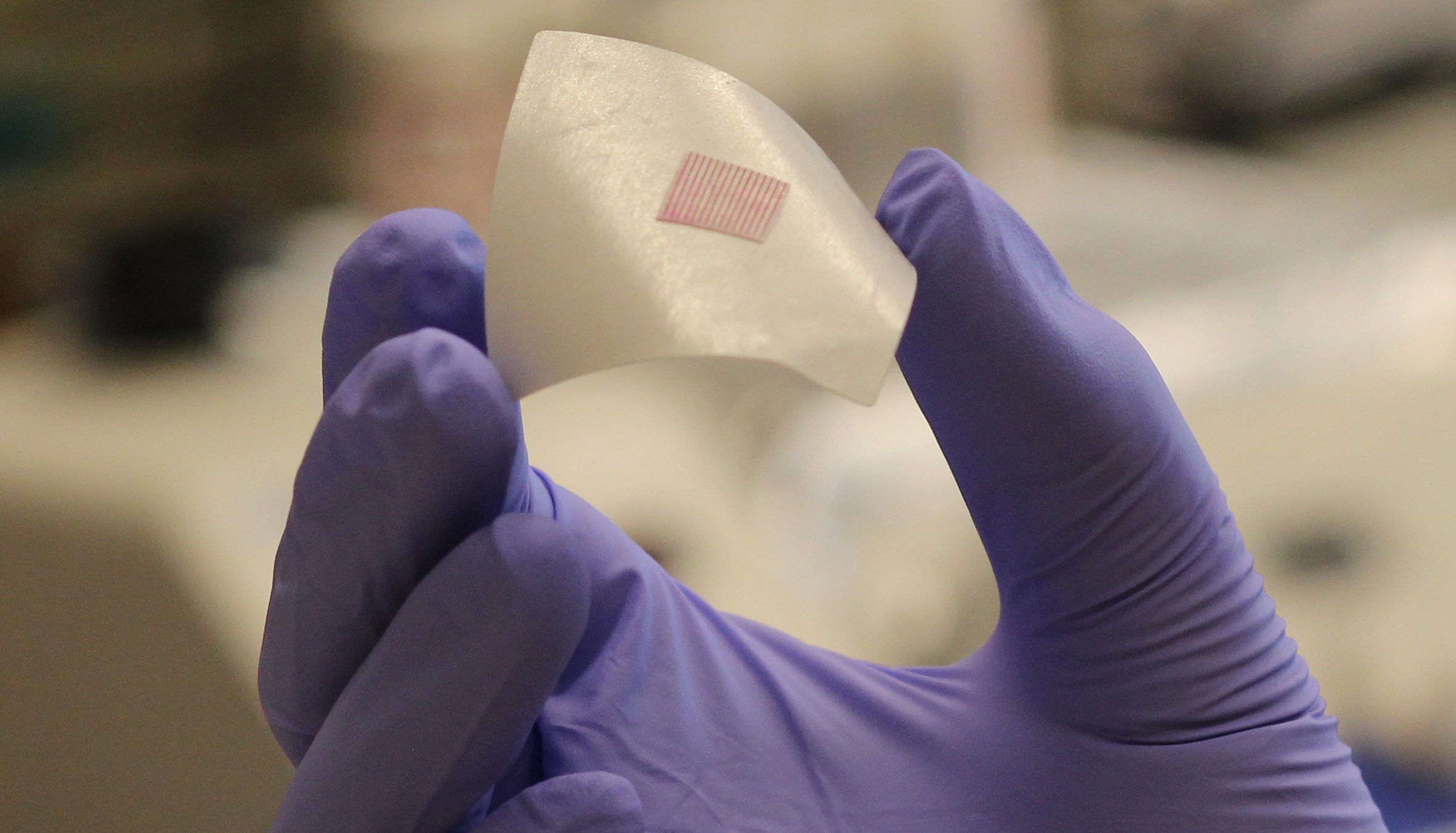
Faculty from UConn recently partnered with the Biomedical Advanced Research and Development Authority (BARDA) to develop a single-use, self-administered microneedle vaccine technology for infectious diseases such as COVID-19, which can be quickly distributed at home in an epidemic or pandemic to provide effective, long-term protection.
Thanh Duc Nguyen, Assistant Professor in the Departments of Mechanical Engineering and Biomedical Engineering in the School of Engineering, and Associate Professor Steve Szczepanek in the Department of Pathobiology and Veterinary Science in the College of Agriculture, Health, and Natural Resources received a $432,990 contract from the U.S. Department of Health and Human Services (HHS) BARDA to develop this technology. In addition to the funding from BARDA, the researchers received $160,000 from the University of Connecticut to share the cost of the project.

Typically, vaccines against infectious diseases like COVID-19 require multiple painful, expensive and inconvenient injections, including a prime and several booster shots. This project will create a self-administered microneedle system which only requires a single-time administration into skin—similar to a nicotine patch—to perform a release profile of vaccines, simulating the effect of multiple injections.
The researchers hope that this single-use technology will keep patients away from visiting health care facilities multiple times during a pandemic. Instead, the microneedle patch can be easily applied at home.
“Our lab has developed a micro array patch (MAP) technology with a one-dose administration of vaccines to replace multiple injections in the conventional vaccination process. Now we hope to use this microneedle system to help patients avoid repeated visits to contagious hospitals in a pandemic like the COVID-19 crisis,” says Nguyen. “We envision that in a pandemic, the microneedle patch can be quickly distributed to people on a global scale for self-administration in their local pharmacies or minute clinics, and potentially even at home, with just one application to create an effective pan-community immune-protection. We are grateful to the School of Engineering and Dean Kazem Kazerounian who provided us seed funding at the beginning to obtain preliminary data for the work.”
The microneedle patch will contain the spike protein, or S-protein on the shell of the COVID-19 virus, and will be programmed to automatically deliver the S-protein as a vaccine antigen against COVID-19 into the skin in time-release fashion —similar to the use of multiple vaccine injections—to trigger the long-term immune protection against the virus.
According to Szczepanek our immune systems tend to either attack or ignore foreign objects such as viruses or food.
In fact, continuous exposure to a foreign object tends to make our immune systems “tolerant” to that object, which is why one doesn’t negatively respond to innocuous things like food (unless one has a food allergy, in which case the immune system is improperly activated).
“Periodic exposure tends to drive the immune system to attack foreign objects, which is why many vaccines against infectious diseases are given as multiple shots over a period of months or years,” says Szczepanek. “One of the holy grails in the field of vaccinology is to develop a platform that can deliver the vaccine with one administration, even if multiple injections are needed to achieve full immunity. We developed the innovation patch which Thanh had engineered. The patch has different needles that dissolve at different times, providing bolus injections of the vaccine at specific times, even though the patch itself is only administered once. This effectively mirrors going to the doctor and receiving multiple shots over a period of months, without all of the hassle or risk of exposure to other people that may be infected.”
Szczepanek adds: “This technology is truly ground-breaking.”
This project is funded in whole or in part with federal funds from the Department of Health and Human Services; Office of the Assistant Secretary for Preparedness and Response; Biomedical Advanced Research and Development Authority (BARDA), under contract number 75A50120C00162 – 210035