Detection of circulating tumor cells (CTCs) in the blood of cancer patients is of intense interest to medical oncologists. The promise of being able to detect such rare cells consistently and reproducibly has attracted considerable attention in recent years.
However, the rarity of CTCs has made this promise difficult to achieve. Limited circulating CTCs has required that blood cells be enriched before detection, and all too often, such enrichment leads to a further loss of CTCs, making them even more rare.
A diagnostic study at the Carole & Ray Neag Comprehensive Cancer Center at UConn Health led by Principal Investigator Dr. Susan Tannenbaum, chief of Hematology-Oncology and service chief of the Neag Comprehensive Cancer Center and Dr. Emily Hsu, fellow in the Hematology-Oncology Fellowship Program, is evaluating CTCs in patients with breast cancer using a novel microscope platform for detection of circulating cells developed by QCDx LLC (Quantitative Cell Diagnostix), a medical device company located at the UConn Technology Incubation Program (TIP) in Farmington, Conn.
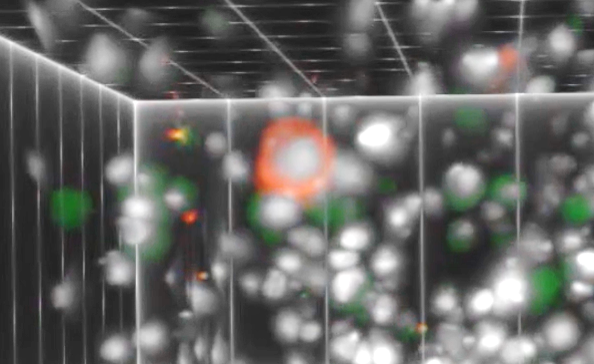
The RareScope™ is uniquely designed to identify and characterize CTCs from blood samples without prior enrichment. In doing so, this technology detects the varied diversity of circulating cells so as to focus treatment, based on biomarkers observed in circulating cells, often different from the cells found in a prior biopsy specimen. The investigation started in July of 2020 and has accrued 21 breast cancer patients with both early and late-stage disease and 13 healthy volunteers.
“In the ongoing CLINBREAC clinical study we have now gathered information on circulating tumor cells using the new RareScope light sheet fluorescent microscopy system to calculate numbers of all circulating tumor cells present in neoadjuvant and metastatic breast cancer patients at multiple time points,” said Tannenbaum. “Additionally, we have been able to quantitate expression of epithelial and mesenchymal markers as well as HER-2, ERα and Trop2 markers by immunofluorescent staining. In our limited data available at this time, we have been able to follow the course of disease with these markers which informs us as to next steps therapeutically. The promising technology has the unique potential to personalize and allow multiple concordant targeted therapies to be engaged that could have significant impact on patient outcomes.”
These results of the ongoing CLINBREAC trial were presented on Dec. 8 at the American Association for Cancer Research SABCS 2021 Symposium in San Antonio, Texas.
The instrument analyzes complete populations of nucleated cells from the patient’s blood sample by 3‑dimensional optical tomography with specialized image analysis software, also involving artificial intelligence.
“Our technology allows the analysis of morphologically intact and/or live cells in immobilized cell suspensions. Without enrichment, every single cell is visualized, and intracellular markers characterized. Rare cells such as CTCs can be detected along with blood cell sub-populations including lymphocytes, that can be critically important for treatment optimization. Furthermore, the technology is available to identify target cells that can be isolated for downstream, single-cell molecular analyses,” said Dr. Triantafyllos Tafas, QCDx Founder and Chief Executive Officer.
Tafas adds: “We believe that our technology is unique in allowing cell-based, mapping of the genomic, transcriptomic and proteomic disease heterogeneity when tissue biopsy is not possible and can critically support the clinicians for improving treatment decisions and benefit the patient’s outcomes and life quality. We are very happy to develop our technology in collaboration with UConn’s Comprehensive Cancer Center and supported by the UConn Technology Incubation Program.”