Changchun Liu, professor of Biomedical Engineering at UConn Health, has developed a new method that improves existing diagnostic technology for a more rapid, sensitive, and deployable approach to molecular diagnostics.
The research, published in Nature Communications, unravels new potential for simple and sensitive nucleic acid detection in various diagnostic settings—including early cancer diagnostics and infectious disease detection— using new and improved clustered regularly interspaced short palindromic repeats (CRISPR) technology. CRISPR technology uses an enzyme that can be programed to seek out a specific stretch of nucleic acid and grab onto it.
PCR-based nucleic acid detection methods have long been regarded as the gold-standard due to their high sensitivity and specificity. However, there is a growing need for alternative solutions that are rapid, cost-effective, and easy to use. CRISPR technology is emerging as a powerful tool for nucleic acid detection for its affordability and simplicity.
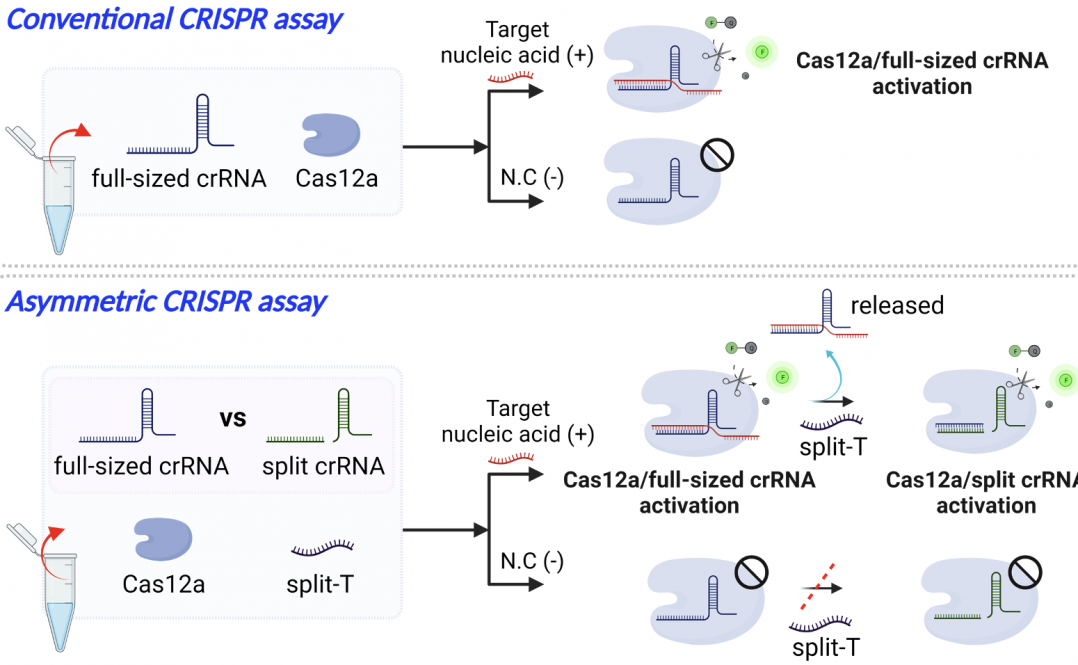
The existing CRISPR method requires two steps—a pre-amplification step of target nucleic acids, and a detection of CRISPR enzymes. While this method is simple and effective than standard PCR-based methods, the two-step requirement and the need for a separate pre-amplification step limits the practical application and lacks quantitative detection ability.
“To meet stringent sensitivity specifications in clinical testing, CRISPR-based nucleic acid detection typically requires target nucleic acid pre-amplification,” says Changchun Liu. “Recently, we have uncovered an asymmetric trans-cleavage behavior of competitive crRNAs in CRISPR-Cas12a reaction. Based on this finding, we developed a sensitive, amplification-free, asymmetric CRISPR assay to quantitatively detect nucleic acids.”
Using CRISPR-Cas12a, an RNA-guided DNA enzyme that can be harnessed for gene editing, the researchers developed a new CRISPR assay by leveraging a unique, competitive reaction between a full-sized crRNA and a split crRNA. The reaction, the researchers found, can induce signal amplification, which can significantly improve the target detection signal—increasing the sensitivity of the diagnostic tool. Additionally, the researchers found that CRISPR-Cas12a can recognize fragmented RNA/DNA targets, which allows researchers to quantitively detect microRNA without the need for the pre-amplification step.
This improved diagnostic technology, without the need for pre-amplification, achieved an attomolar detection sensitivity, 1,000-fold more sensitive than the conventional CRISPR detection.
To test this new technology for efficacy in a clinical setting, the researchers applied the new CRISPR assay to analyze and quantify microRNA-19a biomarker in plasma samples from bladder cancer patients, successfully showing potential for this new CRISPR technology as a powerful tool for simple, rapid, and sensitive liquid biopsy.
“Liquid biopsy is just an example of the clinical applications of our asymmetric assay. We envision that it could have wide clinical implications in early cancer diagnostics and infectious disease detection,” says Jeong Moon, a postdoc researcher in Liu lab and first author of the paper.
Liu is a prolific inventor and UConn’s Technology Commercialization Services (TCS) has filed a portfolio of patents on novel molecular diagnostics technologies developed at Liu’s laboratory.
Ana Fidantsef, Industry Liaison at TCS says, “It is very exciting that this particular amplification-free, CRISPR-Cas12a molecular diagnostic test achieves attomolar-level detection sensitivity, never accomplished before using CRISPR-Cas12a technology. We are eager to reach out to the industry and transfer this technology for commercialization”.
A summary of the technology can be found here.
This work was partially supported by NIH U01CA269147 and U01AI148306.