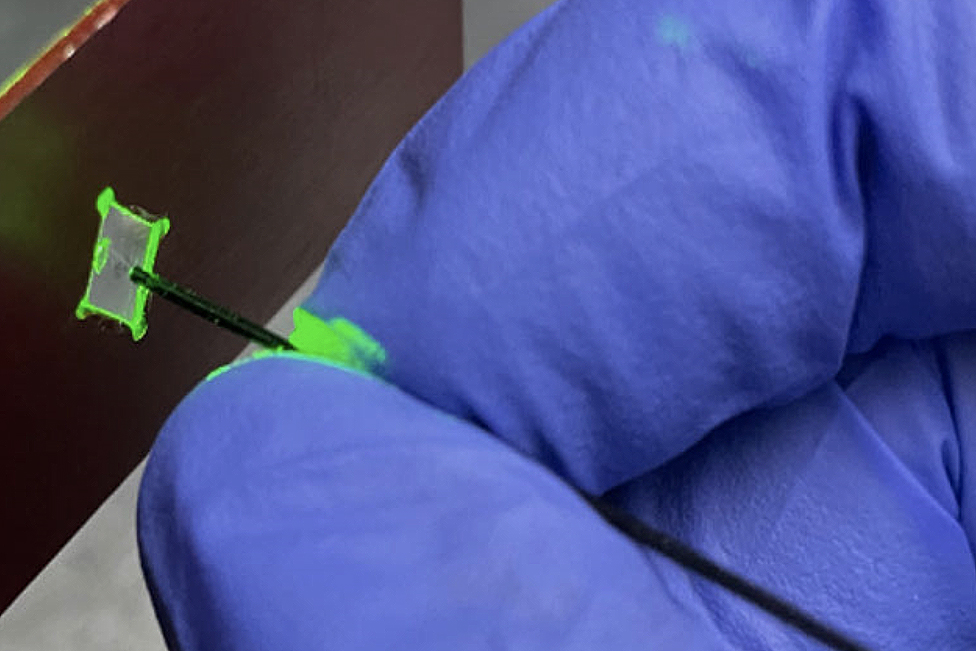
In modern medicine, medical practitioners use endoscopes as an alternative to invasive exploratory procedures and surgeries. At about 2 millimeters in diameter, these camera-tipped tools are fed through the body with a thin cable, typically capturing low-resolution images through a small lens.
But, by using a novel lensless technique, a team of researchers led by UConn Professor of Biomedical Engineering Guoan Zheng can produce exceedingly high-res images with an endoscopic probe as small as the thickness of a human hair.
“To put it in perspective, a typical endoscope image contains about 30,000 pixels. With this new process, we’re able to recover an image with approximately 1.1 million pixels,” Zheng says. “Imagine taking a low-resolution image and adding 40 times more information to it. That’s what we’re achieving.”

In a new study published by Light: Science & Applications, Zheng and his colleagues explain how this new technique, called Synthetic Aperture Ptycho-Endoscopy (SAPE), creates a virtual aperture larger than the physical size of the endoscope, achieving outstanding resolution and visibility. SAPE uses an advanced imaging process called ptychography to capture diffraction— or bending—patterns of light that pass through or reflect off the subject. The “collection” of these patterns—recorded through a bundle of ultrathin optical fibers—results in the ability to construct an extremely detailed, high-resolution image.
“Our goal was to push the boundaries of endoscopic imaging by leveraging the principles of synthetic aperture and ptychography,” Zheng says. “This advancement holds promise for medical diagnostics and industrial inspection, enabling minimally invasive visualization of previously inaccessible regions with impressive clarity.”
The ultra-thin, lensless design of the endoscope allows for minimally invasive imaging of previously inaccessible regions of the body. SAPE can show details as small as 548 nanometers (.000548 millimeters) and can focus clearly over a distance of more than 2 centimeters. This means it provides clear, wide views of complex, curved surfaces and could potentially improve detection and treatment of diseases in fields such as gastroenterology, pulmonology, and oncology.
Unlike a microscope or camera that needs manual or mechanical focusing to produce a clear image, SAPE can do this with light alone. Light waves have two properties: intensity (amount of light) and phase (direction of light). Traditional cameras only capture intensity, losing directional information.
“SAPE recovers both intensity and phase, allowing us to re-trace the path of light,” Zheng explains. “This means we can digitally refocus the image after it’s taken, theoretically to any depth. It’s like having a camera that can focus everywhere digitally, with the ability to choose the focus point after the image is captured.”
Principal Investigator Zheng collaborated on the study with fellow Department of Biomedical Engineering faculty Pengming Song, Ruihai Wang, Jia Liu, Shaowei Jiang, and Bin Feng, along with Lars Loetgering from CarlZeiss, Peter Vouras from the U.S. Department of Defense, Yujin Lee from Yonsei University, Andrew Maiden from the University of Sheffield, and Changhuei Yang from the California Institute of Technology.
“This work is the result of a collaborative effort, and in particular, we’ve been fortunate to work closely with Dr. Bin Feng and his research group. Dr. Feng’s expertise has been instrumental in helping us explore and validate the biological applications of our technology,” Zheng says.
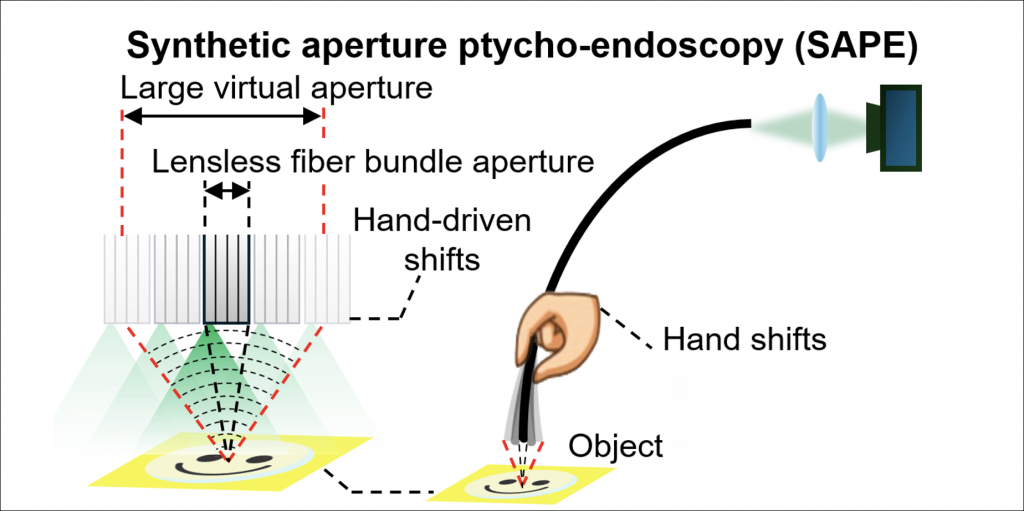
The group developed SAPE with inspiration from the Event Horizon Telescope’s (EHT) groundbreaking black hole imaging in 2019. This remarkable achievement was made possible by using a global network of radio telescopes to synthesize a large virtual aperture. Similarly, Synthetic Aperture Radar (SAR) uses a moving antenna to emit electromagnetic pulses and collect returning echoes, synthesizing a large virtual aperture for high-resolution imaging. The success of these techniques has inspired researchers, like Zheng, to explore the potential of synthetic aperture methods in other fields, including medical imaging.
Zheng and his team continue to refine and expand the capabilities of SAPE. In addition to medical imaging, they’re exploring ways to use SAPE for remote sensing applications with autonomous vehicles and drones.
This project also represents a product of the UConn’s newly launched research center, the Center of Biomedical and Bioengineering Innovations (CBBI).
“The success of this project highlights CBBI’s potential for pioneering research at the intersection of engineering and biomedical innovations,” Zheng explains. “It’s a prime example of how interdisciplinary collaboration within CBBI can lead to innovations that push the boundaries of what’s possible in biomedical imaging.”
This study was partially supported by the National Institute of Health R01-EB034744, the UConn SPARK grant, National Science Foundation 2012140, and the National Institute of Health U01-NS113873.