A new method to detect ribonucleic acid (RNA) without needing any complex lab procedures or heating equipment has been developed by researchers at UConn Health.
Published in Nature Communications, Changchun Liu, professor in the Department of Biomedical Engineering, created a new diagnostic test coined CAARRD (CRISPR Anti-tag Mediated Room-Temperature RNA Detection)—paving the way for simpler detection of viral RNAs such as HIV.
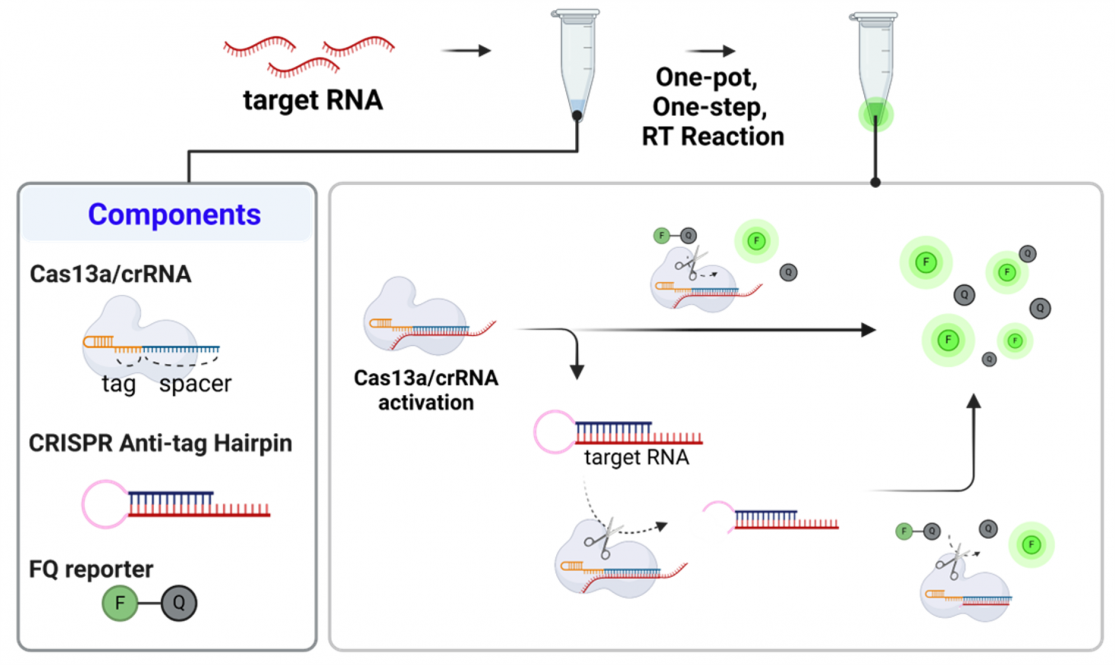
Standard CRISPR/Cas13a tests serve as a powerful tool for RNA detection due to its RNA-targeting capabilities. However, simple and highly sensitive detection using Cas13a faces challenges, such as the need for pre-amplification (or boosting the number of targets) and elevated temperatures.
“With CARRD, we were able to detect extremely small amounts of HIV and HCV RNA—down to 10 attomolar, which is 10,000 times more sensitive than standard CRISPR/Cas13a tests,” says Liu.
In the study, researchers found special “anti-tag” CRISPR sequences that can block the activity of the Cas13a enzyme. The discovery led to the researchers creating the CAARD test—streamlining the detection process.
Since CAARD can operate at room temperature, the test effectively reduces the complexity and time associated with several multiple handling steps.
This discovery paves way for a more simple, sensitive, fast, and cost-effective detection of viral RNAs, such as HIV, that can expand the accessibility of these tests in diverse clinical and environmental contexts.
“We successfully tested the method on real patient samples, detecting HIV RNA from clinical plasma, which shows the potential of this approach for low-cost, accurate viral testing in resource-limited settings,” says Liu.
According to the researchers, future projects will focus on adapting the CARRD test to alternative readout formats, such as paper-based detection or integration with portable, low-cost fluorescence readers to enhance its suitability for point-of-care use in low-resource settings.
This work provides early preliminary data that supported Liu’s recently funded NIH R01 grant.
UConn has also filed a patent application based on this technology.