There are locks and there are keys. And, thanks to a little twist of fate, it looks like UConn researchers have collaborated to find the right key to fit that lock.
The big news is reflected in a cover story in the July 2019 issue of the journal Nanoscale. The lead authors are graduate students, Armin Tahmasbi Rad and Shipra Malik, representing the labs of Dr. Mu-Ping Nieh, Professor of Chemical and Biomolecular Engineering and Dr. Raman Bahal, Assistant Professor of Pharmaceutical Sciences.

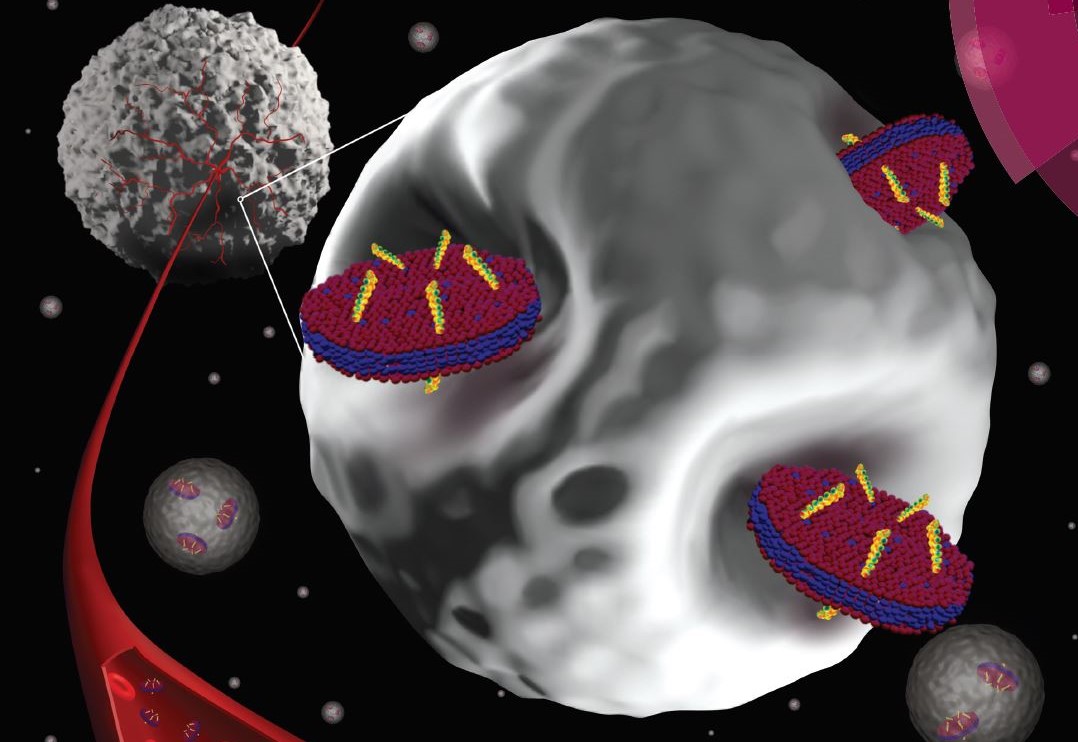
The science is impressive. It involves the delivery of peptide nucleic acids (PNAs) — artificial mimics of DNA that can bind to the target DNA or RNA and are useful in gene therapy and diagnostic techniques — which are synthesized in Bahal’s lab — to the cancerous cells that make up malignant tumors via minute nano-size discs – developed in Nieh’s lab. These nanodiscs are approximately 200 times smaller than the red blood cells that feed a tumor’s growth and they are exactly the right size and shape to penetrate the tumor and deliver the cancer killing PNAs to their targets.
As summarized in Nanoscale, the research demonstrates that the novel platform for PNA delivery holds great promise for controlling gene expression and regulation through improvements in accessibility of molecular targets and increased specificity of cancer fighting agents.
In less scientific terms, you might think of it as the malignant tumor being a lock, and the nanodisc that carries cancer-fighting agents as the key. This discovery has the potential of not only reducing the time it will take to treat tumors – because the nanoparticles will speed to their target and gain entry – but also of reducing the amount of toxins that are released into the human body with many current chemotherapy protocols.
And, while the science itself is impressive, the fact that it comes from two separate labs that normally work completely independently from one another – one located in the Gant Building and one just down the lane in the Pharm-Bio building – really gives a nice twist to the story.
As Bahal tells it, “I got an email from a graduate student telling me he was interested in my research and asking if we could get together. So I said sure, and suggested we meet for coffee.”
That graduate student was Rad, who was nearing completion of his Ph.D. studies in the Department of Chemical & Biomolecular Engineering. A graduate of Tehran Polytechnic University where he earned his bachelor’s degree in biomedical engineering, and of Oklahoma State University where he earned a combined Master’s degree with his home school, Rad had long been interested in the work of W. Mark Saltzman, Ph.D. in Yale’s Department of Biomedical Engineering.
Bahal did post-graduate work in the Department of Therapeutic Radiology at Yale in the lab of Peter M. Glazer prior to coming to UConn in 2018. In that capacity, he had also collaborated with Saltzman, and that intrigued Rad.
“I read that Raman was coming to UConn from Yale where he’d worked on gene therapy and gene targeting,” he says, “and I knew he was doing work I was interested in. Our meeting was really that simple. We just had mutual interests. I needed something to prove that my nanosystem is versatile and effective, and Raman needed a way to get PNAs delivered directly to their targets.”

Once initial introductions had been made, graduate student Shipra Malik quickly came on board. Malik is a Ph.D. student in Bahal’s lab. She earned her Bachelor’s of Pharmacy and Master’s of Pharmacy in Pharmaceutics from the University of Delhi, India and began her Ph.D. studies at UConn in 2018. Her research work is focused on developing antisense therapy based on nanotechnology for targeting tumors.
“Exploring nanodiscs for delivery of PNA was really intriguing,” she says. “I was developing PLGA, an FDA approved polymer-based nanoparticle for PNA delivery in tumor cells. Armin was developing a completely different delivery system which had not yet been explored for PNAs. He was enthusiastic and being from different backgrounds, we were able to bring together our expertise making the research more impactful.
“One of the highlights of the Nanoscale paper is that Armin was working on the formulation and characterization of nanodiscs while my focus was on biological endpoint analysis where I was evaluating the efficacy of this new nanoplatform for PNA delivery.”
Bahal says that the collaboration has been rewarding in many ways. “We are living in an era of precision medicine. Interdisciplinary collaborations are imperative in solving some key issues related to drug delivery for the success of precision medicine.”
He adds, “Our next step is to work on pre-clinical studies to assess the efficacy of these nanodiscs in conjunction with PNA technology.”
As for his part, Nieh is equally enthusiastic. He says, “Our research group has been conducting fundamental research to investigate the formation mechanism and stability of self-assembling lipid nanodiscs for more than ten years. We understand some important parameters that harness the system, but there is still a large area for us to explore. It is a rewarding experience to see our efforts in basic science, in collaboration with Raman’s group, leading to a potential platform for cancer treatment.”
Nieh further explains that the nanodisc under study can be incorporated with other amphiphilic or hydrophobic species through one-pot synthesis [a strategy to improve the efficiency of a chemical reaction] that can be easily manufactured on an industrial scale. “Our ultimate goal,” he says, “is to create a reliable, generalized system for delivering nanodisc carriers to cells.”
Funding for this research was made possible, in part, through a program called the START Preliminary Proof-Of-Concept Fund that supports the preliminary validation of early stage technologies that have possible commercial potential. The money behind START comes from a generous grant provided by the CT Next Higher Education Fund and is administered through UConn’s Office of the Vice President for Research.