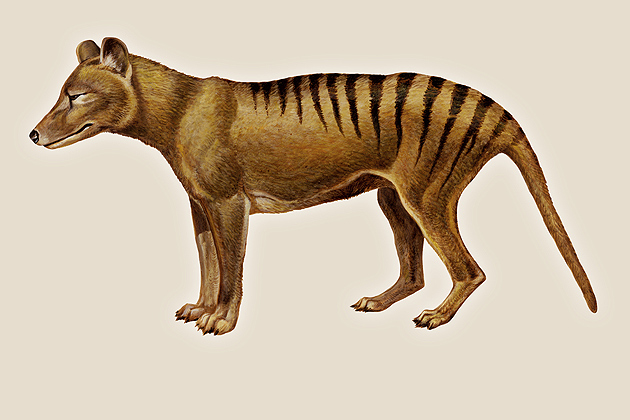
The enigmatic Tasmanian tiger, also known as the thylacine, was hunted to extinction in the wild at the turn of the 20th century, and the last one died in a Tasmanian zoo in 1936.
Now scientists have sequenced a portion of the thylacine genome, showing that like its cousin, the Tasmanian devil, it had extremely low genetic variability. Having poor genetic health makes animals more susceptible to diseases, which can threaten the survival of the species.
The results suggest that both animals’ genetic makeup was affected by their isolation from mainland Australia.
“We found that the thylacine had even less genetic diversity than the Tasmanian devil,” says the study’s senior author, Andrew Pask, associate professor of molecular and cell biology in the College of Liberal Arts and Sciences. “If they were still around today, they’d be at severe risk, just like the devil.”
Pask and Brandon Menzies from the Leibniz Institute for Zoo and Wildlife Research in Berlin, along with co-authors from Australia, published the article today in the journal PLoS ONE.
The thylacine is fascinating to scientists because although it was a marsupial, explains Pask, it looked so much like a dog that even to this day, most archaeologists can’t tell the two skeletons apart.
“This is the most striking example of convergent evolution that we have in mammals,” says Pask. “It was completely unique, so its extinction was a massive loss.”
Named for its telltale stripes, the Tasmanian tiger stood as tall as a medium-sized dog and once roamed across both Australia and Tasmania, which at one time were connected by a land bridge. But after the bridge flooded about 10,000 years ago, the mainland tigers were outcompeted by the introduced dingo and eventually vanished. A government-imposed bounty then drove the tigers to extinction on Tasmania.
Pask and his colleagues speculated that the tiger suffered low genetic diversity because it was geographically isolated from its counterparts in Australia.

Using a combination of traditional and next-generation DNA sequencing techniques, Pask and his colleagues surveyed pelts, bones, and preserved specimens of the thylacine from more than 100 years ago. The scientists found the individuals to be 99.5 percent similar over a portion of DNA that is normally highly variable, and 99.9 percent similar to the tiger’s previously published mitochondrial genome.
These new data suggest that the genetic diversity of the tiger was limited before its extinction. The authors also suggest that, combined with previous data, their study shows that the genetic composition of both the tiger and the Tasmanian devil was likely due to isolation from mainland Australia.
A deadly virus that covers the animal’s face with tumors is currently driving the Tasmanian devil to extinction. Low levels of genetic diversity, says Pask, make the Tasmanian devil even more susceptible to the disease.
“It’s a sad situation, because right now there’s no cure for the tumor,” says Pask. “All we can do is take the populations that are not affected and breed them.”
Pask notes that if hunters had not decimated the thylacine, it might still be around today, but would also be especially susceptible to diseases.
“From a conservation standpoint, we need to know these things about animals’ genomes,” he says. “There are a lot of fragile animals in Australia and Tasmania.”
The next step, Pask says, is to sequence the thylacine’s complete genome.
“We really want to understand how a marsupial can look so much like a dog,” he says.