A new 3-D fabrication technique invented by a UConn engineering professor could provide a safe and convenient way to deliver multiple doses of a drug over an extended period of time with a single injection.

Other 3-D printing techniques have been limited for such applications because they rely on printable inks that are potentially toxic to the human body.
But UConn assistant professor of mechanical engineering Thanh Nguyen circumvented those obstacles by adopting an additive manufacturing technique commonly used for the manufacture of computer chips.
The technique, which Nguyen calls SEAL (StampED Assembly of polymer Layers), can create hundreds of thousands of drug-carrying microparticles made of a biocompatible, FDA-approved polymer currently used for surgical sutures, implants, and prosthetic devices.
During the process, the polymer, PLGA, is shaped into a drug-carrying micro-shell designed to degrade and release its contents over an extended period, ranging from a few days to a few months. This allows for a drug’s release into the body in bursts, similar to what happens when a patient receives multiple injections over time.
“This application could enable the creation of a new set of single-injection vaccines or drugs, which will help avoid the repetitive, painful, expensive, and inconvenient injections often required to administer drugs like growth hormones and pain medicine,” says Nguyen, who invented the technique as a postdoctoral researcher in Professor Robert Langer’s lab at MIT. Nguyen recently left MIT and is a new addition to UConn’s mechanical, biomedical, and regenerative engineering research teams.
Details of the novel technique appear online in the Sept. 14 issue of Science. Langer, the David H. Koch Institute Professor at MIT, and Ana Jaklenec, a research scientist at MIT’s Koch Institute for Integrative Cancer Research, are the senior authors of the paper. Nguyen is a lead author along with Kevin McHugh, another postdoc in Langer’s lab.
“We are very excited about this work because, for the first time, we can create a library of tiny, encased vaccine particles, each programmed to release at a precise, predictable time, so that people could potentially receive a single injection that, in effect, would have multiple boosters already built into it,” says Langer. “This could have a significant impact on patients everywhere, especially in the developing world where patient compliance is particularly poor.”
Langer’s lab began working on the new drug delivery particles as part of a project funded by the Bill and Melinda Gates Foundation, which was seeking a way to deliver multiple doses of a vaccine over a specified period of time with just one injection. That could allow babies in developing nations, who might not see a doctor very often, to get one injection after birth that would deliver all of the vaccines they would need during the first one or two years of life.
An Unconventional Route
Langer had previously developed polymer particles with drugs embedded throughout, allowing them to be gradually released over time. However, for this project, the researchers wanted to come up with a way to deliver short bursts of a drug at specific time intervals, to mimic the way a series of vaccines would be given.
Traditionally, researchers have focused on chemical processes for the sustained release of drugs. But those processes are limited when it comes to developing microcapsules capable of releasing drugs in short, targeted bursts.
With extensive experience in nanotechnology and microelectronics, Nguyen thought he could apply an electromechanical process called photolithography, similar to what is used to make computer chips, to create the core-shell microparticles the lab was searching for.
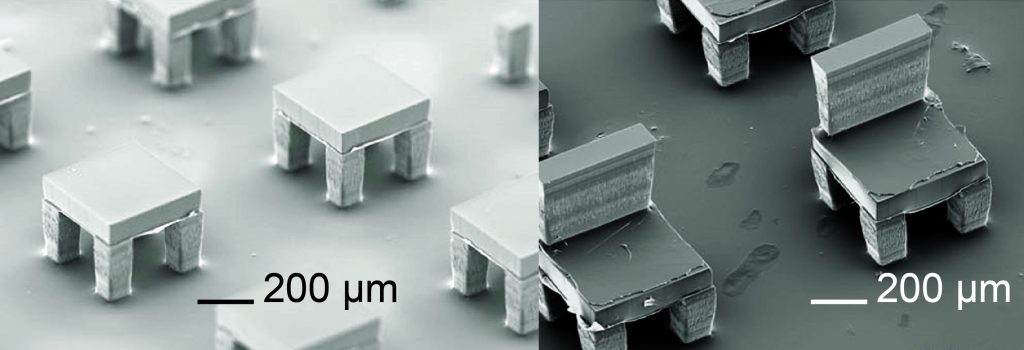
In the SEAL method, particles just a few hundred microns in size are shaped in thousands of silicon molds that sit on a glass slide and look like tiny coffee cups. Nguyen and the research team developed a custom-built dispensing system capable of loading the hollow of each “cup” with a particular drug or vaccine. Separate polymer “lids” are created and fitted on each cup using a novel assembling device, also designed by Nguyen for the SEAL process. A slight amount of heat is applied to seal the lid once the drug is inside.
Nguyen said his lab mates were skeptical of his idea at first, but Langer encouraged him to proceed.
“Although I invented and developed the SEAL method, the achievement was made possible through teamwork from a great group of researchers,” says Nguyen. “Professor Langer encouraged me to pursue an unconventional route to manufacturing drug particles. He also inspired me to develop the method further as a platform technology comparable to 3-D printing.”
MIT research scientist Jaklenec says applications for the new fabrication process go beyond drug delivery.
“Part of the novelty is really in how we align and seal the layers,” she says. “In doing so, we developed a new method that can make structures which current 3-D printing methods cannot. This new method can be used with any thermoplastic material and allows for fabrication of microstructures with complex geometries that could have broad applications, including injectable pulsatile drug delivery, pH sensors, and 3-D microfluidic devices.”
Leon Bellan, an assistant professor of mechanical engineering and biomedical engineering at Vanderbilt University who was not involved in the research, says the approach offers an impressive level of control for constructing 3-D microparticles.
“It’s a new take on a 3-D printing process, and an elegant solution to building macroscopic 3-D structures out of materials that are relevant for biomedical applications,” he says.
Timed Release
The molecular weight of the PLGA polymer and the structure of the polymer molecules’ “backbone” determine how fast the particles will degrade after injection. The degradation rate determines when the drug will be released. By injecting many particles that degrade at different rates, the researchers can generate a strong burst of a drug or vaccine at predetermined time points.
“In the developing world, that might be the difference between not getting vaccinated and receiving all of your vaccines in one shot,” McHugh says.
In mice, the researchers showed that particles were released in sharp bursts, without prior leakage, at 9, 20, and 41 days after injection. They then tested particles filled with ovalbumin, a protein found in egg whites that is commonly used to experimentally stimulate an immune response. Using a combination of particles that released ovalbumin at 9 and 41 days after injection, they found that a single injection of these particles was able to induce a strong immune response that was comparable to that provoked by two conventional injections with double the dose.
The researchers have also designed particles that can degrade and release hundreds of days after injection. One challenge to developing long-term vaccines based on such particles, the researchers say, is making sure that the encapsulated drug or vaccine remains stable at body temperature for a long period before being released. They are now testing these delivery particles with a variety of drugs, including existing vaccines, such as inactivated polio vaccine, and new vaccines that are still in development. They are also working on strategies to stabilize the vaccines.
“The SEAL technique could provide a new platform that can create nearly any tiny, fillable object with nearly any material, which could provide unprecedented opportunities in manufacturing in medicine and other areas,” Langer says. These particles could also be useful for delivering drugs that have to be given on a regular basis, such as allergy shots, to minimize the number of injections.
Other authors on the paper are Allison Linehan, David Yang, Adam Behrens, Sviatlana Rose, Zachary Tochka, Stephanie Tzeng, James Norman, Aaron Anselmo, Xian Xu, Stephanie Tomasic, Matthew Taylor, Jennifer Lu, and Rohiverth Guarecuco.
This work was funded by the Bill & Melinda Gates Foundation OPP 1095790. Fellowship support for Kevin J. McHugh was provided by the NIH under Ruth L. Kirschstein National Research Service Award (F32EB022416), and for Thanh D. Nguyen by the Max Planck Society and Alexander von Humboldt Foundation.
Watch video of the microcapsule filling and sealing processes here and here.



