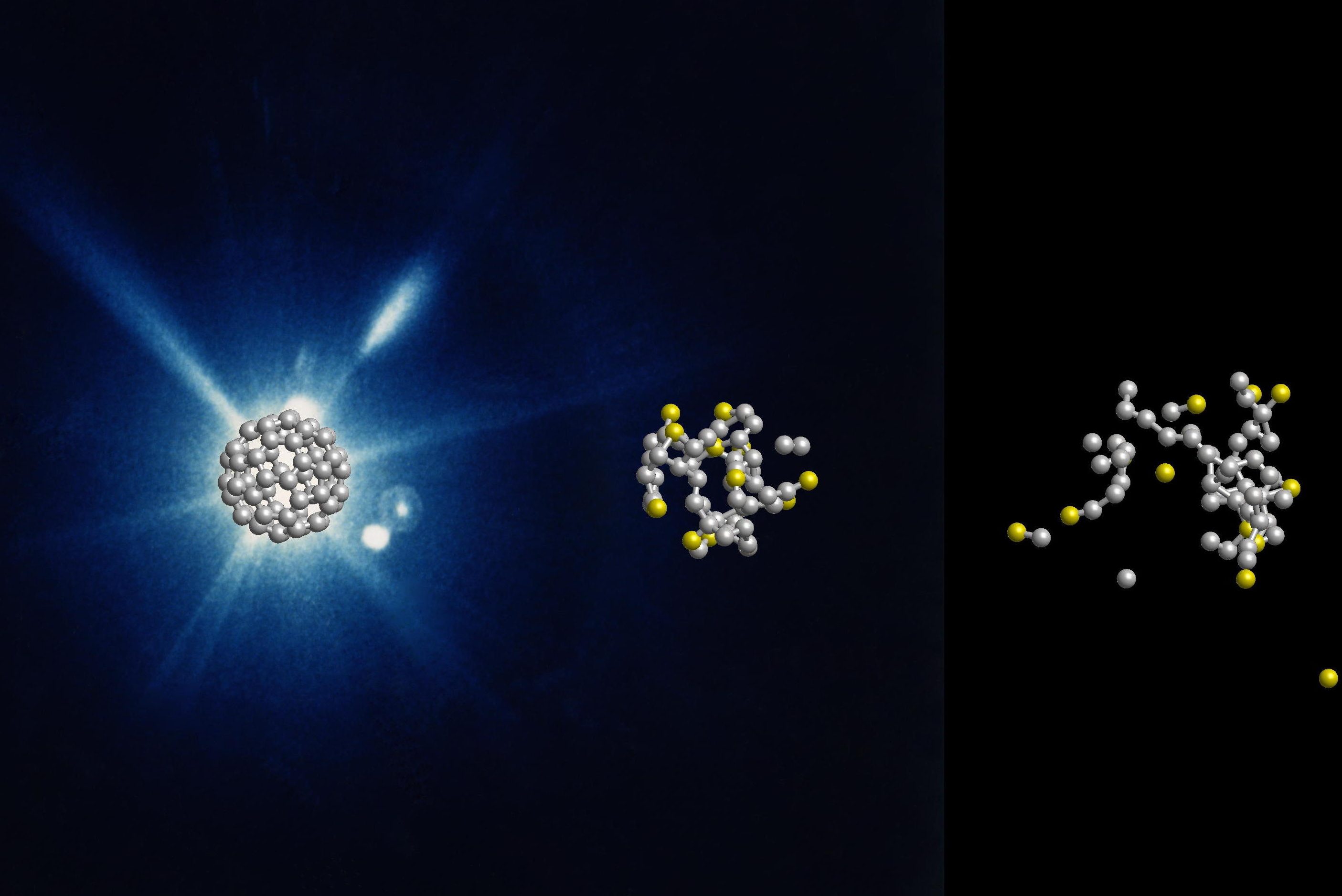
An international research team has observed in real time how soccer ball-shaped molecules made of carbon atoms burst in the beam of an X-ray laser. The molecules disintegrate more slowly and differently than expected, they found.
The results mean it will be easier than expected to capture X-ray images of biological molecules, a process that may reveal some of life’s defense mechanisms against radiation, says the team led by Nora Berrah, professor of physics at the University of Connecticut, and published in the most recent issue of the journal Nature Physics.
Chemical reactions inside of living cells happen quickly. Really, really quickly. It can take just a few femtoseconds (a femtosecond is one quadrillionth, or 0.000000000000001, of a second) for an enzyme to slice another molecule, for example. Until recently, there has not been a way to observe the process directly; there’s no camera with a flash that fast.
But now, the Linac Coherent Light Source at SLAC National Accelerator Laboratory in California produces bright X-rays pulses fast enough to catch the molecules in the act of reacting to light. “X-ray lasers have opened up the ultra-fast and ultra-small world,” says Berrah.
The researchers were experimenting with buckminster fullerenes, or buckyballs for short. These spherical molecules consist of 60 carbon atoms arranged in alternating pentagons and hexagons like the leather coat of a soccer ball.
“Buckyballs are well suited as a simple model system for biomolecules,” explains Robin Santra, who is a lead scientists at DESY at the Center for Free-Electron Laser Science in Germany and a physics professor at the Universität Hamburg. “Since they consist of only one type of atom and have a symmetrical structure, they can be well represented in theory and experiment. This is a first step before the investigation of molecules from different types of atoms.”
Using the X-ray laser Linac Coherent Light Source, the scientists shot short X-ray flashes of about 20 femtoseconds duration at individual buckyballs and observed their effect in real time with a temporal resolution in the range of about ten femtoseconds. The data show that the X-ray flash knocks electrons out of about one in five of the 60 carbon atoms. After that, nothing happens for some time. Only after a few dozen femtoseconds do carbon atoms gradually detach from the molecule.
Since the fragmentation of the buckyballs on this time scale is not explosive but happens gradually, the researchers speak of the evaporation of the neutral atoms. The experimental data could not have been recorded without an X-ray laser and sophisticated instrumentation and they were interpreted with the help of expert theoretical modelling.
The whole process takes about 600 femtoseconds. This is still unimaginably short by human standards, but extremely long for structural analysis with X-ray lasers.
“Our work showed that radiation damage is slowed down due to intrinsic physical processes,” says Berrah. The implications to the field of ultrafast molecular imaging is that chemical structure is resistant to the intense electromagnetic environment created by the X-ray free electron laser. This delayed fragmentation should be true of most other complex molecules, and means that it will be easier to image such molecules with this technique than we originally anticipated, Berrah says.
For the structural analysis of proteins, researchers usually grow small crystals from the biomolecules. The bright X-ray laser flash is then diffracted by the crystal lattice and generates a typical diffraction pattern from which the spatial structure of individual proteins can be calculated. The spatial structure of a protein reveals details about its exact function. Protein crystals are very sensitive and evaporate from the X-ray laser flash. However, previous investigations had shown that the crystal remains intact long enough to generate the diffraction image before evaporating, and thus can reveal its spatial structure.
The new study now confirms that this is also the case with individual molecules that are not bound in a crystal lattice.
“Our findings with buckyballs are likely to play a role in most other molecules,” the scientists emphasize. Since many biomolecules are notoriously difficult to crystallize, researchers hope to be able to determine the structure of ensembles of non-crystallized proteins or even individual biomolecules with X-ray lasers in the future.
“We are now studying even larger systems, small molecules inside cages made of 80 carbon atoms, to understand the transfer of electrons between the molecules and the cage, mimicking natural processes in biomolecules,” Berrah says.
The study involved researchers from UConn, Imperial College London, SLAC National Accelerator Laboratory in California, University of Gothenburg, University of Texas, French Synchrotron Soleil, Kansas State University, Max Planck Institute for Nuclear Physics, Tohoku University in Japan, University of Potsdam, Max Born Institute, Universität Hamburg and DESY. CFEL is a joint institution of DESY, the Max Planck Society and the Universität Hamburg.
The research was funded by the Chemical Sciences, Geosciences, and Biosciences Division, Office of Basic Energy Sciences, Office of Science, U.S. Department of Energy, under grant No. DE-SC0012376.