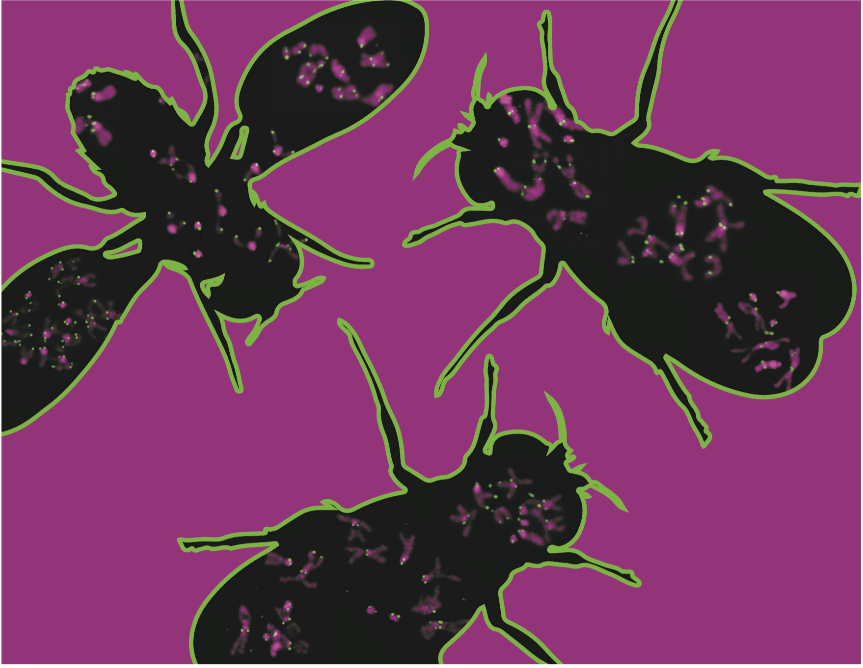
Classic pictures of DNA show it as a collection of X- and Y-shaped blobs called chromosomes. Centromeres are the little hubs at the center of those chromosomes, holding them together and helping them get properly inherited during cell division. Now, UConn cell biologist Barbara Mellone, her student Jason Palladino, and colleagues report in the cover article of the 10 February issue of Developmental Cell that they were able to make fake centromeres that fooled cells into rearranging their chromosomes.
When one species diverges into two, the new species sometimes change the arrangement of their DNA. New centromeres develop naturally for the newly rearranged chromosomes. Mellone’s team found they could replicate that by inserting synthetic centromeres into living cells. These researcher-made centromeres generally wreaked havoc, breaking and reforming chromosomes and harming the whole organism.
The synthetic centromeres were made up only of the proteins typically found in centromeres. They didn’t contain any DNA like a real centromere would, suggesting that the proteins are enough to tell a cell where centromeres need to form. Most of the time a centromere’s role might be purely structural. But during cell division, the DNA in a real centromere might have an important role to play. They aren’t quite sure what that role is, but Mellone, Palladino, and their fellow researchers are working on it.