Huntington’s. Parkinson’s. Muscular dystrophy. Lou Gehrig’s. These diseases share a common cause that devastatingly robs sufferers of their energy, muscle control, and in the case of Huntington’s, their sanity. But now, a group of researchers from UConn describes how a possible therapy might work.
What all those fearsome diseases have in common is dysfunctional mitochondria. Mitochondria are the body’s tiny power plants. These minuscule, rod-shaped structures inside our cells take in oxygen and nutrients and put out ATP, the body’s fuel (ATP is to cells what gasoline is to cars.) When mitochondria don’t work so well, the dysfunction can cause strange and awful symptoms that are particularly distressing in parts of the body that require lots of energy: particularly muscles, the brain, and nerve tissue.
Mitochondrial diseases tend to worsen with age. Scientists have guessed that mitochondria age as the rest of our body does. Damage acquired over time may contribute to mitochondrial diseases, but they aren’t entirely sure what’s happening or how to stop it.
“They’re insidious diseases because they rob your cells of their energy. They’re so hard to diagnose and the symptoms can be so diverse,” says Nathan Alder, a molecular biophysicist in the Department of Molecular and Cell Biology at UConn.
Alder and other researchers from UConn, the University of Texas, and Alexandria LaunchLabs are researching a group of compounds that seem to protect and even repair damage to mitochondria. The researchers describe the compounds, called SS peptides, and one potential way they may work to heal mitochondria in an upcoming issue of the Journal of Biological Chemistry.
SS peptides are made of amino acids, the building blocks of proteins, but each SS peptide is only four amino acids long. They all have the same basic plan: two amino acids with a positive charge alternating with two aromatic amino acids (“aromatic” is a chemistry term meaning they have a ring-like structure similar to benzene).
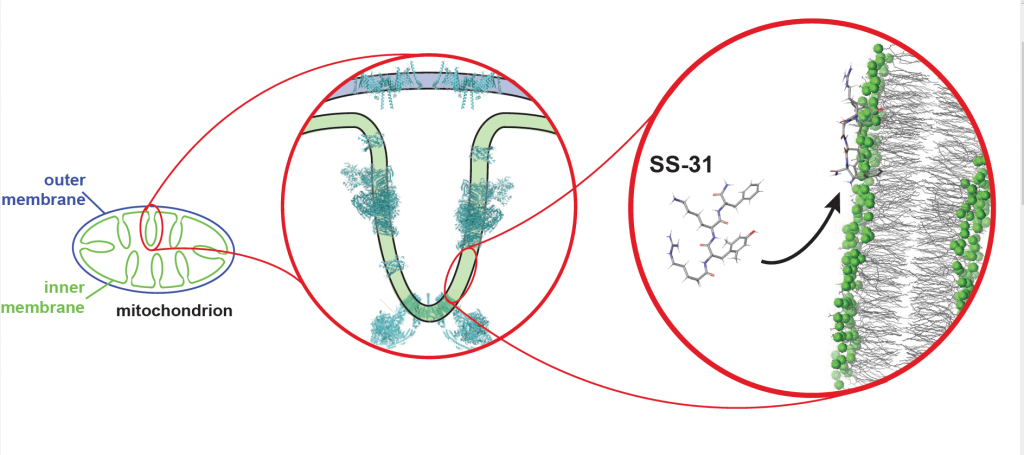
Previous research by Hazel Szeto at Cornell University, who first described SS peptides and served as co-author on this study, showed that SS peptides can enter into any cell in the body, and mitochondria suck them up like sponges. Alder and his colleagues wanted to figure out what the peptides were doing when they got in there. Using approaches ranging from computer modeling to studying mitochondria in the lab, they began to see the peptides’ effects. It looks like they can alter and potentially repair mitochondria by tuning the electric properties of their membranes.
Mitochondrial membranes are intricately creviced double-layers of fatty molecules called lipids that surround proteins sticking out of the membrane itself. The outer layer of the membrane “talks” to the rest of the cell, sensing conditions and passing ATP and other molecules back and forth. The labyrinthine inner layer of the membrane holds the ATP factories. One of the special lipids enriched in the inner membrane, cardiolipin, has a strong affinity for SS peptides.
Mitochondria tend to accumulate positively charged things like calcium ions—mitochondria actually serve as storage centers for cellular calcium. Yet calcium overload can cause damage to mitochondria’s cardiolipin-containing membranes over time, ripping into the membrane and causing permanent damage.
SS peptides can prevent that from happening, Alder and his colleagues found. The peptides are positively charged but in a gentler way than calcium; they snuggle up against the mitochondrial membrane and shield it from the smaller, more damaging calcium ions.
“This is probably not the only effect of SS peptides. But it’s an interesting one,” Alder says. The researchers want to understand more about how the peptides interact with the mitochondria and why they appear to have such broad-based efficacy against so many mitochondrial disorders. The team is currently using UConn’s nuclear magnetic resonance facilities to get detailed pictures of SS peptide structural features and how the peptides might alter or maintain the shape of the mitochondrial membranes. “We know they work. We want to know how they work. By understanding the mechanism of action, we can engineer more effective peptide analogs and possibly tailor them to treat specific mitochondrial afflictions,” Alder says.