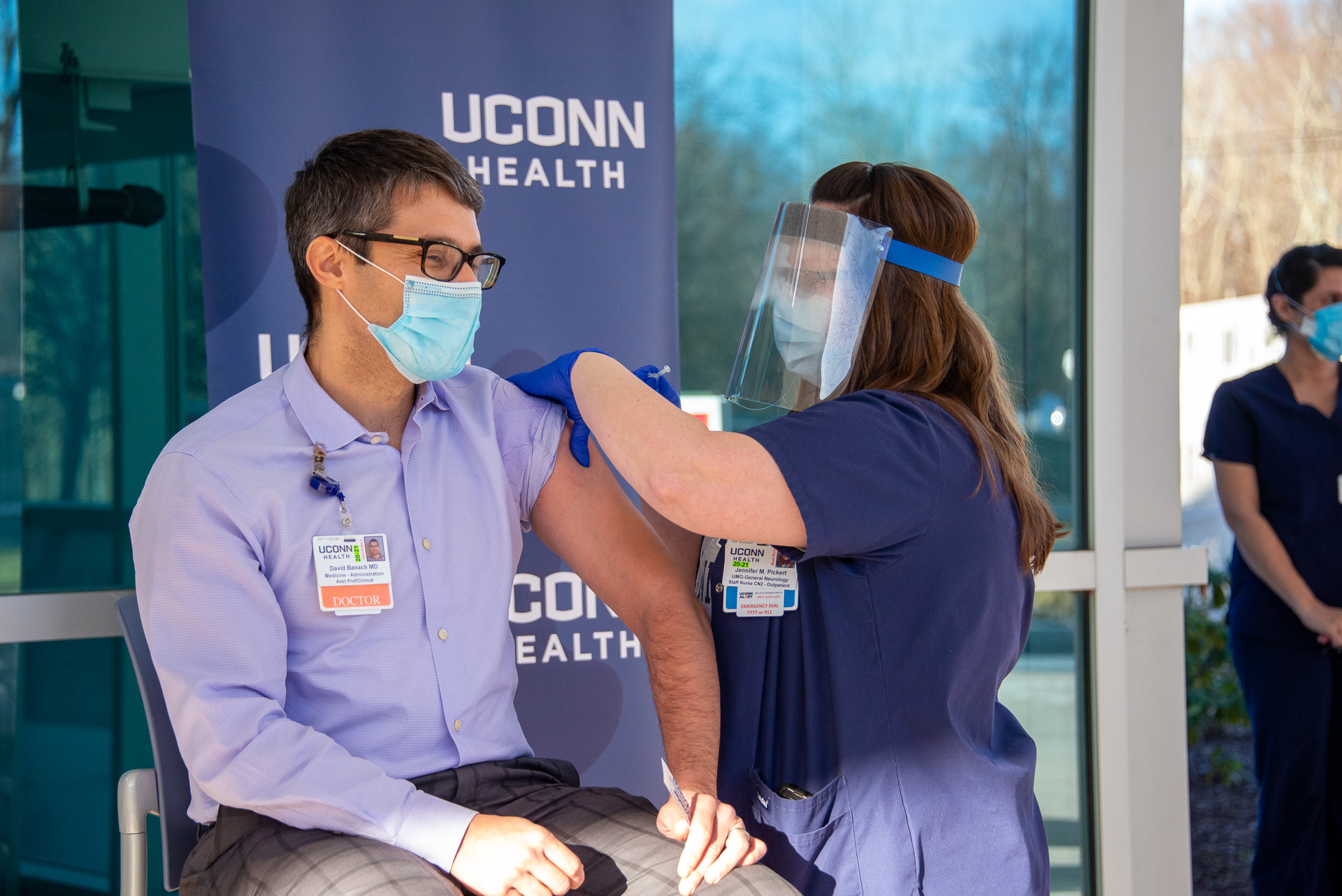
The initial doses of the first approved COVID-19 vaccine are here, and health care workers are part of the group that’s getting it first. One of them is Dr. David Banach, UConn Health infectious diseases physician and hospital epidemiologist, who sees the vaccine not only as a major breakthrough in the fight to end the pandemic, but also as an opportunity for his clinical colleagues to lead that effort and set the tone for the rest of the world. Here are some key facts about the COVID-19 vaccine, with Dr. Banach providing explanations of each:
The COVID-19 vaccine won’t infect you with COVID-19.
“There is no live virus in this vaccine, so you can’t actually get infected with SARS-CoV-2 from the vaccine. What this vaccine has is messenger RNA, which is a little bit of genetic code that allows the body’s natural machinery to make the protein that will generate an immune response.”

You may actually want some side effects from the COVID-19 vaccine.
“You might get some soreness at the injection site, maybe some fatigue for a day or two, but that can be a good thing, a sign your body is making that immune response. That’s what is going to protect you in the future if you get exposed to the virus. The data from the clinical trials show the side effects – the soreness, fatigue, in some cases a short-lived fever – occur the first few days afterwards, and the rate of serious side effects is extraordinarily low for this vaccine.”
The vaccine was developed relatively quickly, but not by compromising the scientific process.
“When you look at Operation Warp Speed and how this process moved really quickly, that was really focused on the research and development piece and the manufacturing piece. Importantly, the phase 3 clinical trial was not rushed. This is the same type of clinical trial that we would do for any other vaccine. We followed people for at least two months. The clinical trials were huge, and they had diverse populations. So that part of the whole process wasn’t rushed at all, and that’s the most important part.”
Developing immunity through vaccination is better than through actual infection.
“COVID-19 infection can cause severe illness, but even among some individuals who get mild illness, there may be a long-term sequelae. This virus behaves differently than any other virus that we’ve been up against in terms of the unusual effect that it has on different organ systems, as well as blood vessels, and it’s unique in that sense. There’s concern about long-term effects from it, and that’s justified, but I think we won’t know the full details until we follow patients over time and get more data.
“This vaccine is the way to generate the immunity without getting the viral infection and being at risk for those long-term consequences. I think that’s all the more reason to really encourage vaccination. The more people who get the vaccine, the better. It’s hard to put an exact number on what percentage of the population we need vaccinated to achieve herd immunity, because it’s a bit more nuanced, and estimates are based on statistical models that have limitations. The bottom line is that you want a really high percentage of the very vulnerable population to get vaccinated, like the nursing home residents and other the people living in congregate settings where the virus can travel like wildfire and cause very high rates of severe illness. But you’d also want the general population to be vaccinated a very high rate as well, if you’re really going to prevent transmission from occurring in the community.”
Don’t throw out those masks just yet.
“We know this vaccine prevents people from developing symptomatic and severe COVID infection. I think what we don’t know is the effect it’s going to have on viral transmission, including asymptomatic shedding of virus. For instance, people who get the vaccine might still potentially shed virus, potentially at a lower level. The vaccine will prevent them from actually becoming ill, but vaccinated individuals might still be able to have virus in their nose and their respiratory system.
“I think with time, we’ll know the effects of vaccination on viral shedding and spreading the virus as we get more data. In the meantime, sticking to those preventive measures – masking, distancing, and avoiding group activities – is going to be really important, especially until the whole population gets vaccinated, and that’s going to take at least a few months.”
It’s wise to get vaccinated even if you previously were infected with COVID-19.
“There were some people included in the clinical trials who were previously infected, and we’re still collecting data on those individuals. I think what we can say is that there weren’t any safety concerns about the people who had previous infection and then received the vaccine. Thus, the CDC does recommend that people who were previously infected with COVID-19 receive the vaccine.
“What we do know is that there has been a very small number of individuals who have had reinfection, so it can happen. And there’s going to be some waning of the immune protection over time, and this is an important area of ongoing study. That’s why I think while we are learning more, while battling this surge of the pandemic, vaccinating those individuals who have been infected is going to be important.”
Immunity from the vaccine is not instantaneous.
“The COVID-19 vaccine clinical trials using the Pfizer-BioNTech and Moderna mRNA vaccines were designed using a two-dose series in order to generate the optimal level of protection from the vaccine. That’s why getting both doses of the vaccine is essential. Although there is likely some individual variability, immunity may not be optimal until several days after the second dose. The phase 3 clinical trials used a period of at least one-to-two weeks after the second dose as a marker of immunity during which they were able to demonstrate the efficacy of the vaccines in protecting against COVID-19 infection.”
Listen to Dr. Banach discuss this topic here: