Stem cells replenish the body by spawning fresh, young cells that can turn into skin, or muscle, or any other tissue. But how stem cells do that—while remaining stem cells themselves—has been a mystery. Now UConn Health researchers have a solution, reported in the 30 March issue of the Proceedings of the National Academies of Sciences.
Eggs are one of the many things stem cells can make. Fruit flies, for example, constantly make new eggs.
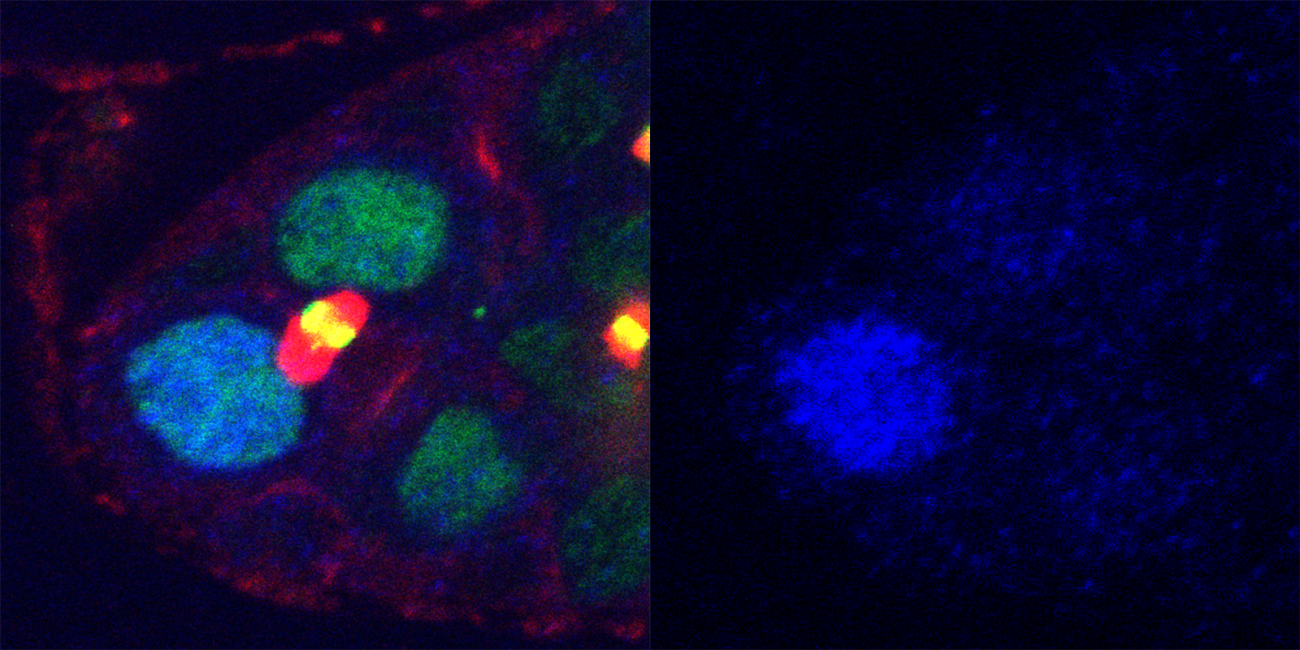
“We were looking at fruit fly ovaries. Germ-line stem cells in fruit flies produce eggs all the time,” UConn School of Medicine cell biologist Mayu Inaba says. The stem cell “divides into two different cells, a stem cell and an egg.” Inaba and her colleagues wanted to know how a single stem cell could divide into two different kinds of cells, side-by-side.
For clues, they got Mad. Mad is a protein that scientists already knew to be important for maintaining stem cells. When the protein Mad is in a stem cell, it’s active; in a normal cell, it’s not.
Inaba and her colleagues discovered that, during stem cell division, activated Mad becomes more concentrated in a “daughter cell” that is going to stay a stem cell than in its sister that will become an egg. This happens even before the cells completely separate and wall themselves off from each other. This difference could explain why one stays a stem cell and one becomes an egg.
But how did the cells know to have more or less active Mad? An enzyme called phosphatase is able to deactivate Mad inside a cell. Intriguingly, Inaba found that phosphatase was equally common in both cells. So why did the two cells have different amounts of active Mad that led to their very different fates?
To understand how it is possible, UConn School of Medicine physicist Boris Slepchenko used Virtual Cell, a computational framework developed at UConn Health’s Center for Cell Analysis and Modeling, to model the distribution of active Mad between daughter cells. The models helped them see a curious thing: even when the daughter cells were still connected and proteins could move back and forth freely, different levels of active Mad were emerging in these cells.
The models also helped explain why: separation in space of the enzymes that activate Mad, and the enzymes that deactivate Mad (phosphatases), sets up activated Mad gradients, and their steepness is sharpened by phosphatase activity. That a tiny difference in positions of opposing enzymes of only a few micrometers–like tapping a break just a tiny bit in one of the cells–could change the cells’ fate was quite surprising to the scientists.
Inaba and Slepchenko’s future research will continue investigating how positioning controls cell destiny.