Dr. Cato T. Laurencin, University Professor and Albert and Wilda Van Dusen Distinguished Endowed Professor of Orthopaedic Surgery, has developed synthetic artificial stem cells (SASC), a new class of stem cells that may avoid traditional pitfalls associated with stem cell therapies.
These synthetic cells can be tailored to specific tissue types and used across systems to provide immunomodulatory and regenerative benefits. The findings were published in the Proceedings of the National Academy of Sciences.
There have been three general classes of stem cells: embryonic stem cells, adult stem cells (like those found in fat), and induced pluripotent stem cells, which come from non-stem cells that are reprogrammed to behave like stem cells. Now there is a fourth class: SASC cells.
A critical function of stem cells has been their ability to release bioactive compounds that can foster regeneration. Recent studies have shown that these compounds, rather than the cells themselves, may be the most important factor in determining if a stem cell treatment is effective.
These compounds are paracrine factors, also known as the secretome. They can diffuse across cell membranes and spark changes in neighboring cells. This is known as the paracrine effect. It is responsible for cell differentiation during development as well as regeneration and repair in adult tissues.
Using the secretome allows scientists to tailor the therapy to the patient’s needs and has wider applicability across tissue types. Yet, the secretome still faces challenges including time-intensive cell maintenance.
SASC cells are a promising novel solution to these problems. With SASC cells, scientists can choose the type and amount of different biological factors to achieve a specific purpose in a certain tissue type. This effectively mimics the paracrine effect without using secretome or stem cells.
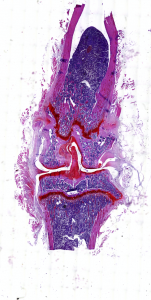
Laurencin and his team developed and tested SASC cells for the treatment of osteoarthritis. They programmed the artificial synthetic cells to secrete factors that are most important for treating the condition including anti-inflammatory compounds and those that slow joint narrowing, a hallmark of arthritis.
He compared the effectiveness of SASC cells against a current state-of-the-art treatment, stem cells derived from adipose tissue (ADSCs). They found SASC cells had similar effects to ADSCs in many domains, and were more effective at eliciting an anti-inflammatory response.
This discovery opens the door for future advances in regenerative therapy, especially given that due to their small size, SASC cell injection can be a minimally invasive procedure.
Further, since these cells are artificial and synthetic, they don’t face the same risks as stem cells. With other stem cells, the paracrine effect may be different than desired based on the properties of the donor cells. SASC cells are also significantly less likely to prompt an adverse immune response in the patient.
While this study focused on osteoarthritis, SASC cells’ ability to be tailored means they can theoretically be applied in the treatment of many other conditions.



