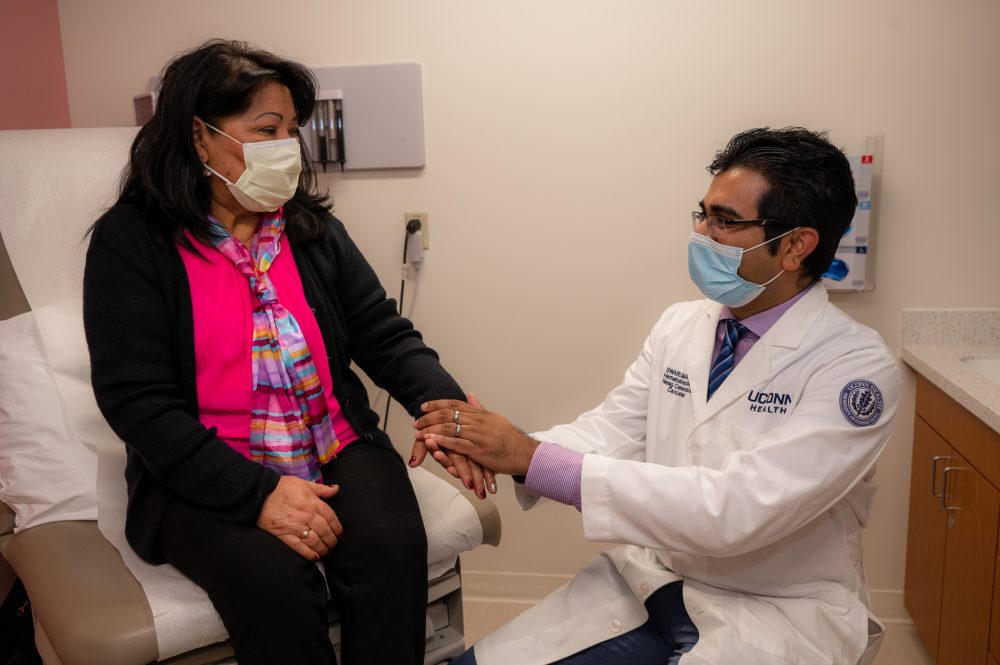
After five years of chemotherapy and other treatments for her relapsing and refractory multiple myeloma, Soledad Cruz wasn’t going to let cancer define her, but she was at the end of her treatment options.
That’s when Dr. Swarup Kumar, assistant clinical professor of Medicine, at the Carole and Ray Neag Comprehensive Cancer Center at UConn Health introduced her to a new drug recently approved by the Food and Drug Administration (FDA) under its accelerated approval program, and first given commercially by Kumar in the United States.
UConn Health was the first to obtain this treatment as well as administer it to a patient. Cruz was the second patient at UConn Health and in the state of Connecticut to have received the treatment and just a few weeks later, she has undetectable myeloma markers in her blood.
Multiple myeloma is a type of blood cancer where there is an overgrowth of plasma cells found predominantly in the bone marrow. In multiple myeloma, these abnormal plasma cells, a type of white blood cell, form tumors in the bones that results in significant bone damage. Other parts of the body, such as the kidneys, are also affected, which can result in rapid kidney failure if left untreated. In almost all patients, the cancer eventually comes back, or relapses, after treatment. With each new treatment, the cancer becomes less likely to respond, and the time before the disease comes back gets shorter.
Those with advanced multiple myeloma have an aggressive clinical course with their tumors and are resistant to other forms of treatment. Such patients have very few treatment options and usually only live for a short period of time.
Obtaining the Medication
Kumar, who leads the plasma cell disorder program which encompasses multiple myeloma, was tracking the clinical trials of the new treatment, teclistamab (Tecvayli), and the positive data piqued his interest early on. Teclistamab was already shown in a landmark clinical trial to be effective with durable treatment responses in people with multiple myeloma for whom previous therapies have not worked.
Teclistamab is a monoclonal antibody that binds to receptors on cancer cells and immune cells, in what is otherwise known as a bispecific T cell engaging monoclonal antibody (BiTE). This dual binding activates an immune response and directly results in killing of the myeloma cells.
While the FDA gave accelerated approval to teclistamab on Oct. 25, 2022, to treat adults with multiple myeloma that has recurred after at least four prior lines of treatment, the process to fast-track obtaining the medication at UConn Health as soon as it was approved started months earlier.
The conversation began between Kumar and Dr. Doug Hackenyos, pharmacy oncology clinical coordinator at UConn Health, during the previous summer, without any timeline yet from the FDA as to when it would be approved, although the clinical trial data had already looked very promising.
Kumar introduced Hackenyos to the medical science liaison from the Janssen Pharmaceutical Companies of Johnson & Johnson, the company that created the medication. This connection was pivotal in laying out what needed to be accomplished in advance to make sure that, once the FDA approved the drug and it became commercially available, UConn Health was ready to move forward in providing it to patients.
Multiple myeloma progresses fast, so we don’t have time to wait. — Dr. Swarup Kumar
Due to the side effects and safety concerns with the drug, a Risk Evaluation and Mitigation Strategy (REMS), a drug safety program that the FDA requires to help ensure the benefits of the medication outweigh its risks, is required by any prescriber and hospital administering the medication. The REMS is designed to reinforce medication use behaviors and actions that support the safe use of that medication. While all medications have labeling with information about medication risks, only a few medications require a REMS.
Hackenyos worked with his team to get the required registration, set up specific procedures, and make sure the staff was trained appropriately under the REMS requirements. In addition to the REMS requirements, he worked alongside EPIC Willow and Beacon teams for the electronic builds required, so that when the specific data became available, it could be quickly incorporated, and some pieces that usually take upward of two or more weeks after a drug is physically available could already be in place.
When the FDA approval came through in October, UConn Health was initially quoted 4-6 weeks before the drug would be available on shelves. Soon after that, the representative let Hackenyos know the company was accelerating its timeline, and the order would be available that week.
“The accelerated timeline for drug availability required us to ramp things up quickly to make sure all the systems were in place,” says Hackenyos. “There are lots of little details – the nuts and bolts – and safety steps behind the scenes to make sure we can use these medications safely and effectively.”
The Patient
Cruz, 67, from East Hartford, had beat cancer before. She had breast cancer in 2010 and spent five years in remission. In 2015, she started having pain in her leg and saw her primary care practitioner. She sought out several treatments including physical therapy and massage, but nothing was helping the pain that was getting increasingly worse.
In 2017, she had a bone marrow biopsy that determined she had multiple myeloma in three places: her back, hip, and leg. The tumors on her leg had damaged the bone so significantly that it had become brittle, and she needed a rod to be placed on her femur.
Multiple myeloma causes many symptoms, but excessive tiredness and bone pain are often the first people notice. Other symptoms include weakness in the arms and legs and/or a sensation of numbness in the arms and legs which can occur when the tumor affects the bones in the spine, causing them to collapse and press on the spinal cord.
Cruz began treatment with radiation and chemotherapy, and in June of 2018 was referred to Smilow Cancer Center where she spent 30 days in the hospital for a stem cell transplant. She did not go into remission following that treatment and had to start back on chemotherapy.
She spent five years having various combinations of chemotherapy treatments and other medications including a steroid that was having a serious effect on her mood.

“I was going crazy, I was depressed, angry, and arguing with everyone, and that was not me,” says Cruz.
Once she stopped that medication those issues went away.
In 2020, she was referred to Kumar at UConn Health because he specialized in multiple myeloma. He started her on an infusion of chemotherapy four times a week. This made her sick.
“There were days I wouldn’t want to get up,” says Cruz.
Her myeloma light chain cancer markers went down lower than 150, so she went to visit family in Peru, while in Peru her numbers jumped back up to 1,400. Upon her return, Kumar started her on a second chemotherapy treatment. That treatment made her ill, and she ended up in the hospital for 12 days in the Intensive Care Unit.
At this time, she and her family were focusing on a good quality of life with her five children and five grandchildren and visiting family in Peru.
This past fall was a tough one for Cruz: in September she had pneumonia, in October it was shingles, and in November she contracted COVID-19. She went four months without chemotherapy because she was on so many other medications. Her numbers again went up from 170 to approximately 584 during that time.
“She recovers and pulls through. I don’t know how she does it each time, but she does. Something in her is fighting through and she is not going to let go,” says Jessica Torres, one of Cruz’s daughters.
Kumar told her at that time about the new drug teclistamab and that she qualified to receive it. She and her family researched the information provided by Kumar and decided she should try it.
The Treatment
Kumar has so far treated seven patients with teclistamab since its approval, including those referred from other hospitals in the state. In fact, the first three patients to receive teclistamab in Connecticut were at UConn Health. The treatment requires the patient to stay in the hospital for 10 days in a dedicated unit with specially trained staff to ensure proper monitoring and management of side effects, particularly that of cytokine release syndrome and neurologic toxicity.
Before starting treatment with teclistamab, patients usually receive an antiviral medication to prevent shingles reactivation during treatment, as well as immune globulin infusion to decrease the overall risk of infection. An antiseizure medication is also added for the first several days on treatment preventatively, due to the potential for neurotoxicity during initial treatment course.
About 1 to 3 hours prior to receiving the first doses of teclistamab, patients receive several medications to help reduce the risk of reactions and cytokine release syndrome including a corticosteroid, an antihistamine, and a painkiller and fever reducer.
Teclistamab is administered in the form of an injection under the skin, usually in the stomach area or thigh. The first “step-up dose” is administered on day one, a second “step-up dose” with a higher amount of teclistamab is usually given between days two and four after the first dose, and the first “treatment dose” with a fixed amount of teclistamab is administered two to four days after the second step-up dose. Subsequent treatment doses are administered once a week and usually don’t require a hospital stay.
Before and periodically during the treatment blood tests will be performed to monitor for side effects.
“The drug is tricking the immune system to make it think that an infection is happening in the body. By binding the immune cells to cancer cells, immune cells are activated and recruited in large numbers to fight and kill myeloma cells. This is one of the reasons why cytokine release syndrome (CRS) is often expected with this treatment. The occurrence of CRS is actually letting us know that the drug is working the way it should,” says Kumar.
“The first symptom of CRS is a fever which can quickly progress to more severe symptoms such as low blood pressure, decreased oxygen levels, or even organ failure if not promptly identified and treated,” Kumar says. “The good news with this treatment is that the vast majority of patients only experience mild grade CRS which usually doesn’t recur after the first few doses of treatment. CRS symptoms can safely and effectively be managed by the utilization of drugs such as tocilizumab and corticosteroids and often don’t require escalation of clinical care. At UConn Health, we have a dedicated team of individuals with expertise in CRS and neurologic toxicity related to these types of treatments who are involved in the treatment from the moment patients are first evaluated in our facility.”
After the initial treatment, patients continue to receive weekly injections of the medication.
“At this time the medication is used to extend patients’ lives. While there is not enough information to know if patients will go into long-term remission, the treatment appears very promising. A substantial number of patients are actually able to achieve a deep and durable response that they may eventually allow us to even stop therapy and monitor,” says Kumar. “A number of similar treatments (bispecific antibodies) are currently in development for multiple myeloma patients, and it is going to be important for us to understand how effective such robust anti-cancer therapies are going to be earlier in the patient’s cancer treatment when their immune systems are fitter.”
“I did not have any major reactions to the medication, I have no more pain, so much more energy and I am happy,” says Cruz. “I want to spread the word and let others know of this new option to help them.”
Cruz is now having the treatment every other week. She has regular blood work done and currently has undetectable myeloma markers in the blood after only a few weeks of the drug. She will have another bone marrow biopsy done in a few months.
Kumar credits the effort of all his team members and also the speed with which things happened at UConn Health once the medication was approved. No hospital in the country received the medication as quickly.
“Multiple myeloma progresses fast, so we don’t have time to wait,” says Kumar.
Those interested in learning more about this treatment can contact the Carole and Ray Neag Comprehensive Cancer Center at UConn Health at 860 679-2100 and ask for the malignant hematology nurse navigator. Dr. Kumar is actively accepting patients who may qualify for this medication.