Chronic skin wounds that never heal can be disfiguring and painful. Now, in the journal Biomaterials, University of Connecticut bioengineers describe a special scaffold for new skin that also kills bacteria. Their invention has the potential to help many people heal, and to prevent new chronic wounds from developing in the first place.
Chronic wounds are wounds that heal slowly or not at all. They can be caused by burns or other trauma, or by complications of diabetes and kidney disease. And such wounds are usually exacerbated by infections. Chronic wounds afflict about 8.2 million people in the US according Medicare data, and many more worldwide. The economic cost to society is in the tens of billions of dollars, and the human cost is even higher: for example, diabetic foot ulcers, a common type of chronic wound, have a five-year death rate of around 30%.
Despite the enormous burdens chronic wounds cause, there are few good treatments available. And the chronic infections in these wounds are painful to treat and difficult to eliminate.

UConn biomedical engineer Thanh Nguyen and colleagues from UConn, the UConn School of Medicine, Boston University, and New York University have developed a biodegradable bandage that can cover the wound and encourage new skin to grow while simultaneously discouraging bacteria. The Department of Biomedical Engineering at UConn is a shared department with the UConn School of Dental Medicine, School of Medicine, and School of Engineering.
“Wound healing is a complex process. The blood flow gets disrupted, and clots. Then inflammation occurs to kill off any bacteria in the wound. Then there is a switch to regeneration,” Nguyen says. That is how it works in typically healing wounds. But in chronic wounds, there’s always infection, which causes never-ending inflammation, and no regeneration can occur. The body, due to impairments from diabetes or other conditions, can’t overcome the infections. Doctors can manually debride the infected tissue—meaning they scrape it with a scalpel—but it’s quite painful and not always effective.
“The slow healing rate of chronic wounds enhances the risks for infections and the occurrence of an infection slows down healing even further, leading to a painful cycle. The dressing we developed enhances wound healing and prevents infections simultaneously, which should bring the patient out of this cycle,” says Ritopa Das, a Ph.D. student in bioengineering and first author of the paper.
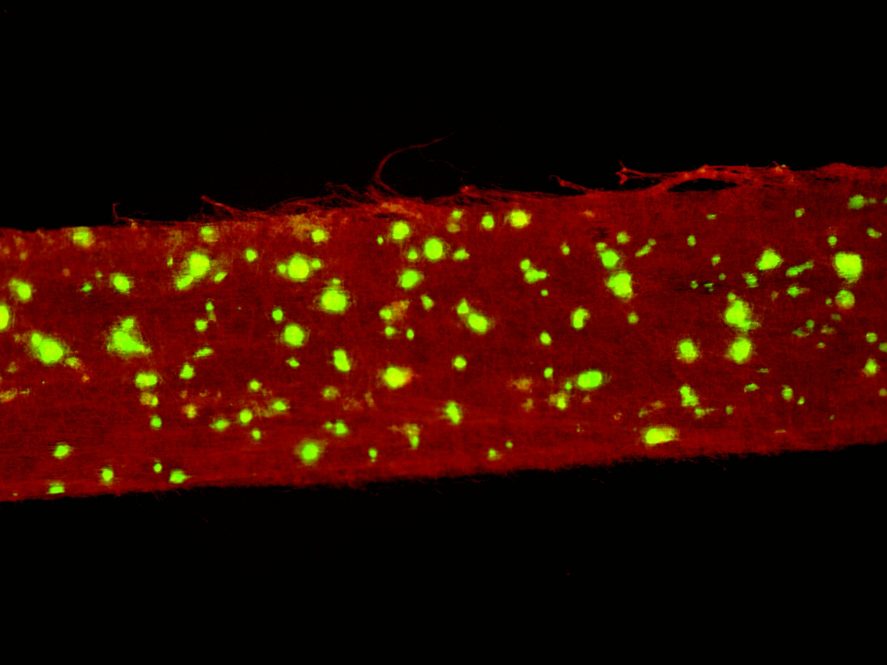
The biodegradable bandage developed by Nguyen and his colleagues is made of a polymer that generates electrical charge when flexed (the researchers used a once-a-day ultrasound to do this.) The polymer is woven so that the side touching the wound has a positive charge, which encourages skin cells to regrow. The side facing out has a negative charge, which reacts with water to create molecules that kill bacteria. And the nano texture of the polymer allows skin to actually grow into it. The non-toxic polymer will slowly dissolve into the body, eliminating the need to remove the bandage and damage the new skin.
The device healed large wounds in mice faster than the closest commercially available treatment. The researchers hope to test it in larger animals soon.