For nearly 30 years, there have been no new medicines to treat stroke patients, but UConn is testing a small-molecule drug in its laboratories shown to reduce damage and restore function after stroke.
UConn School of Medicine has received a follow-up research grant award of more than $2 million from the NIH’s National Heart, Lung, and Blood Institute (NHLBI) to further advance UConn’s testing of its promising stroke drug discovery.
Inventors and principal investigators of the experimental, brain-permeable, anti-inflammatory therapy are Rajkumar Verma, M.Pharm., Ph.D., assistant professor of neuroscience at the Calhoun Cardiology Center, and Dr. Bruce T. Liang, cardiovascular physician-scientist at UConn School of Medicine.
“This renewed NIH grant funding will enable us to further advance our laboratory testing and ultimately apply to the FDA for an Investigational New Drug (IND) application. If approved, it will lead to first-in-human testing,” says Liang.
The NIH’s initial phase 1 funding allowed this collaborative UConn research team to screen for and discover the experimental chemical that has been proven effective in animal models to be both neuroprotective and heal the brain damaged by a stroke by reducing inflammation.
The innovative stroke therapy getting closer to human clinical trials inhibits an important receptor, P2X4, implicated in ischemic stroke damage. This novel P2X4 receptor inhibitor works by stopping and reducing the expansion of brain damage caused by a stroke – the leading cause of disability in the U.S. – to combat its long-term, debilitating effects, such as paralysis of one side of the body, memory loss, speech, language, depression, and vision problems.
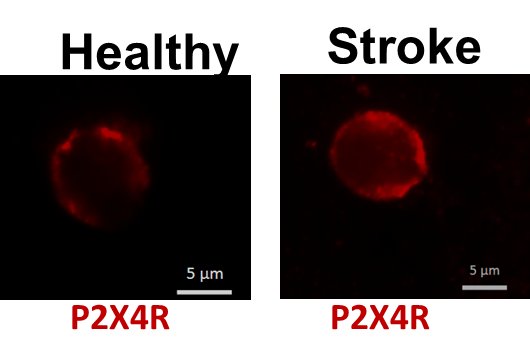
Most strokes are ischemic, which occur when a blockage in an artery leading to the brain causes damage or death of brain cells because of reduced blood flow and oxygen supply. The damaged or dying brain cells release excessive amounts of stored adenosine triphosphate (ATP), a molecule that serves as a danger signal, leading to over-stimulation of its receptor P2X4 (P2X4R), mainly found on immune cells of the blood and brain. When P2X4R is overactive, it causes a cascade of detrimental effects in brain cells, leading to a large stroke.
“Our medication crosses the blood-brain barrier to reach the brain and heal it by blocking the receptor implicated in ischemic stroke damage. It also reduces the brain damage that a stroke inflicts, enhances the possibility for both short-term and long-term stroke recovery and restored function, while expanding the time window available for stroke treatment,” says Verma.
If soon proven successful in animal models for safety and then human clinical trials, the research team believes this neuroprotective drug intervention would have a groundbreaking impact on the future of stroke patient care.
This innovative UConn research, in collaboration with NIH’s Kenneth Jacobson, Ph.D., was initially supported by the NIH via a small business “STTR phase 1 grant: A New Anti-inflammatory Therapy for Ischemic Stroke” grant to the UConn Technology Incubation Program (TIP) start-up company Provascor Pharmaceuticals.
According to the NIH, this follow-up phase 2 grant award’s objective is to continue UConn’s innovative research and development efforts of the drug initiated in phase I with larger, renewed funding based on the already promising results, along with the scientific and technical merit and commercial potential of this new medicine.
The UConn researchers look forward to presenting their research findings to the FDA in the foreseeable future, says Verma.
The team’s latest research findings were recently published in the Journal of the American Heart Association (JAHA).