Unique to UConn School of Medicine is the launch of a clinical trial funded by the National Institutes of Health aimed at enhancing the lives of those with hypothyroidism.
Having a thyroid that doesn’t produce enough thyroid hormone (hypothyroidism) is a very common but disabling condition. Symptoms may include weight gain, fatigue, and muscle weakness. Hypothyroidism is also associated with high cholesterol and increased risk for cardiovascular disease.

The new three-year clinical trial is being led by Francesco S. Celi, MD, MHSc, professor and chair of the Department of Medicine who serves as the James E.C. Walker Endowed Chair at UConn School of Medicine.
The clinical trial designed by Celi is now actively recruiting 90 volunteers with hypothyroidism to study them over a 6-month span each to test the effectiveness of different therapies for the treatment of underactive thyroid.
“Patients with hypothyroidism tend to do well on synthetic hormone replacement called lexothyroxine (LT4), but research shows a sizable number up to 40% still complain of symptoms which can be attributed to hypothyroidism despite having the thyroid stimulating hormone (TSH) within normal range,” stresses Celi. This is possibly caused by the loss of production of T3, the active form of thyroid hormone from the underactive gland.
People develop an under active thyroid when their thyroid gland loses the ability of producing thyroid hormones T4 and T3. Importantly, while T4 is produced exclusively by the thyroid gland, T3 which is the active form of the hormone, is mostly produced by conversion of T4 into T3 in various tissues of the body, with only a small amount actually secreted by the thyroid gland. As a result, to treat the body’s underproduction of thyroid hormone a synthetic medication form of T4 is needed known as levothyroxine (LT4). The efficacy of the synthetic hormone replacement therapy is measured by keeping the body’s TSH in the normal range, because this hormone is highly sensitive to minimal changes in thyroid hormone levels.
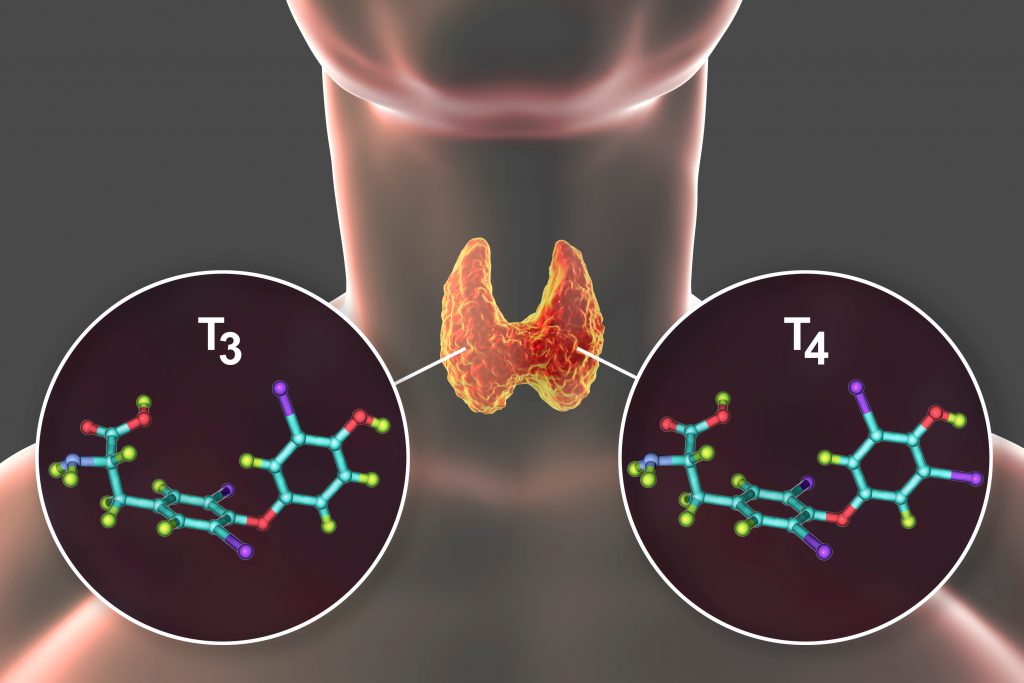
“Our preliminary research data suggest that patients with hypothyroidism, especially those post-thyroidectomy surgery, develop weight gain and increase in cholesterol changes that can be prevented or minimized with combination therapy of synthetic T3 (LT3) and T4 (LT4),” Celi shares.
The study’s goal is to closely examine this phenomenon and help patients reduce their body weight, serum cholesterol, and improve their quality of life. Eligible study participants are those who are 18 and over and on stable thyroid replacement therapy. They will receive free outpatient care for hypothyroidism, study medications, and thyroid hormone and cholesterol lipid analyses for the duration of the study.
In addition, they will have a 2:1 chance to be assigned to a combination of levothyroxine and liothyronine (synthetic T3) to replicate the normal production of thyroid hormones.
UConn’s study is designed to answer three questions: Is the combined use of LT3/LT4 effective in reducing cholesterol and weight in patients with hypothyroidism when compared to LT4 alone?; If so, what is the optimal LT3 dose?; and is LT3 administered once a day as effective as LT3 administered twice daily?
“Thyroid diseases are very common conditions that disproportionally affect women, especially those who are of reproductive age. Also, later in life, there is a greater increase in women experiencing hypothyroidism too,” shares Celi. “What is very interesting is the thyroid disease affects patients in a unique way, and it is incumbent on us discovering which individual would benefit from certain treatments.”
Along with Principal Investigator Celi, study coordinators for this innovative clinical trial are Mallory Edrich and Nicole Glidden of the Department of Medicine at UConn School of Medicine.
Those interested in participating in the new clinical trial can contact the study coordinators at: 860-679-8511 or 860-679-4647.