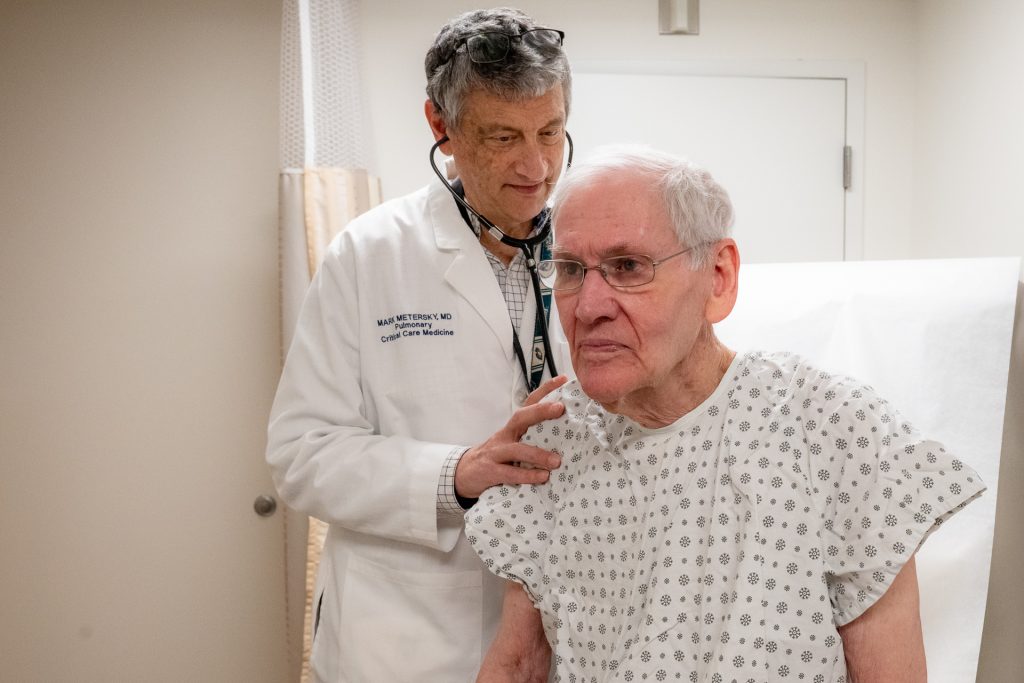
Woody Exley, 85, has been a long-time bronchiectasis patient of UConn School of Medicine’s Dr. Mark Metersky at UConn Health for over 16 years.
“He’s a national leader!” Exley says about Metersky, chief of the Division of Pulmonary, Critical Care and Sleep Medicine, who directs the dedicated Center for Bronchiectasis Care at UConn Health, managing the care of patients like Exley living with the chronic, inflammatory lung condition.
Exley credits his wife with discovering Metersky and his bronchiectasis expertise at UConn Health just a few minutes drive away from their West Hartford home.
Symptoms of bronchiectasis can include chronic cough with sputum (mucous) production, shortness of breath, and fatigue, with acute exacerbations of the debilitating condition causing worsening cough and sputum production, often with fever, shortness of breath, or chest pain, impairing patients’ quality of life. Severe exacerbations may result in hospitalization and even permanent loss of lung function.

“Dr. Metersky is wonderful. He changed my life,” says Exley.
In the late 1990s, Exley kept getting fevers with repeat bouts of pneumonia. But he did not know why until he was finally diagnosed with bronchiectasis, a condition that impacts up to 500,000 Americans, but is often misdiagnosed or has a delayed diagnosis, as it can present similarly to other pulmonary conditions such as COPD or asthma.
Exley was living on a rotation of general antibiotics until he began seeing Metersky in 2009.
“Since I found Dr. Metersky at UConn I have never had another fever,” says Exley. “Dr. Metersky’s care is magic.”
During clinical visits over the years to the Center for Bronchiectasis Care at UConn Health, Exley would simply share sputum samples. If test results identified a certain bacterium brewing in his lungs, Metersky would take action to prescribe the best, targeted antibiotic medication for fighting against that specific bug. Exley also learned how to better cough and clear sputum from his lungs to reduce his risk of lung infection too. Plus, some nebulizer drug treatments have also helped benefit him.
“Dr. Metersky has been taking such good care of me,” Exley says. “He really listens to me and does what’s needed. I owe him a good debt of gratitude.”
This October, UConn Health’s Center for Bronchiectasis Care joined the national Bronchiectasis and NTM Care Center Network that facilitates access to high-quality, specialized patient care for bronchiectasis and NTM lung disease. The Center is now designated by the Bronchiectasis and NTM Association as a Bronchiectasis and NTM Care Center. There are 58 designated centers across the United States. Metersky is also a member of the network’s Steering Committee, comprised of leading experts in the field.
Bronchiectasis Patient Giving Back to Others
When Metersky would ask Exley to consider participating in clinical trials, Exley says, “I was happy to do it.”
And the most recent clinical trial Exley participated in was a big success for him and hundreds of thousands of bronchiectasis patients across the U.S. in need of a treatment.
In June 2021, Exley began participating in a global, randomized Phase III clinical trial testing an experimental drug then called brensocatib compared to placebo.
“They wouldn’t tell me if I was getting the real deal” for the randomized clinical trial he says, but Exley reports he was feeling good during it.
“But now, I have the real deal,” he shares. “The experimental drug is FDA-approved now!” This means Exley now has a prescription for the daily pill.
This achievement was made possible by unique strengths at UConn Health: expert physicians like Metersky leading the science, a dedicated Clinical Research Center supported by the School of Medicine, and patients like Exley who generously volunteer to participate in clinical trials.
In fact, more than 1,700 study participants, spanning five continents, enrolled in the global trial, helping lead to the publishing of very positive clinical trial results about the drug in April’s New England Journal of Medicine. The results led to August’s approval of the new medication as the first-ever FDA-approved treatment for bronchiectasis. Insmed, Inc., the drug’s manufacturer, sponsored the clinical trial.
Clinical trial results showed that the medication targets inflammation and significantly lowers the annualized rate of exacerbations in patients while also slowing their loss of lung function. Also, nearly half the patients in the clinical trial remained exacerbation-free one year later.
“This drug will help many patients living with bronchiectasis and improve their quality of life. This medication is now providing hope for so many patients suffering with daily symptoms,” says Metersky, who in addition to being PI for UConn Health’s clinical trial site, was senior author of the NEJM study and was on the Steering Committee for the global, multi-center clinical trial.
In addition to patients like Exley, the contributions and commitment of Sheila Thurlow, MSN, RN, the CRC’s dedicated study coordinator, were also instrumental to the success of the medication’s Phase III trial at UConn Health.
“This is an exciting, first-of-its-kind drug for the care of bronchiectasis. We are so excited for the FDA-approval of this Brinsupri (brensocatib) that we helped study in clinical trial at UConn Health. This is exactly what we do at our Clinical Research Center,” says Thurlow.
“Dr. Metersky is a great principal investigator who is so well-seasoned, well-versed, and well-known. It was a wonderful experience and privilege working with him on this impactful, global clinical trial that is changing lives for the better,” Thurlow says. “It was a pleasure working with Woody. His consistent compliance and positive presence not only impacted the integrity of the study but made each visit a joy,” adds Thurlow.
Exley says he really enjoyed working with the CRC and Thurlow for the clinical trial study, too.
“The success of our Phase 3 trial was truly a team effort, and I’m incredibly proud of the Clinical Research Center staff for their dedication and professionalism throughout the study. Sheila Thurlow, our research nurse coordinator, was the glue that held everything together – ensuring protocol adherence, supporting participants, and keeping our team aligned every step of the way. Her leadership and attention to detail were instrumental in the success of the trial at UConn Health, which contributed meaningfully to the high-quality data that supported the FDA’s approval of this important new therapy,” says Elizabeth Laska BSN, RN, CCRC, nurse manager of the Clinical Research Center at UConn.
“I feel I made a contribution,” says Exley. “I would definitely recommend other patients choose to participate in clinical trials. It’s really important for people with health issues to contribute to making progress for future treatments.”
He concludes, “I am happy the drug has been approved! I am hoping it has a positive effect on others.”
The CRC of UConn School of Medicine is currently providing support for over eighty open clinical trials. Learn more at: health.uconn.edu/clinical-research-center.



